Primary care visit use after positive fecal immunochemical test for colorectal cancer screening
- PMID: 28621809
- PMCID: PMC5643012
- DOI: 10.1002/cncr.30809
Primary care visit use after positive fecal immunochemical test for colorectal cancer screening
Abstract
Background: For some patients, positive cancer screening test results can be a stressful experience that can affect future screening compliance and increase the use of health care services unrelated to medically indicated follow-up.
Methods: Among 483,216 individuals aged 50 to 75 years who completed a fecal immunochemical test to screen for colorectal cancer at a large integrated health care setting between 2007 and 2011, the authors evaluated whether a positive test was associated with a net change in outpatient primary care visit use within the year after screening. Multivariable regression models were used to evaluate the relationship between test result group and net changes in primary care visits after fecal immunochemical testing.
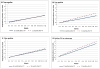
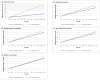
Results: In the year after the fecal immunochemical test, use increased by 0.60 clinic visits for patients with true-positive results. The absolute change in visits was largest (3.00) among individuals with positive test results who were diagnosed with colorectal cancer, but significant small increases also were found for patients treated with polypectomy and who had no neoplasia (0.36) and those with a normal examination and no polypectomy performed (0.17). Groups of patients who demonstrated an increase in net visit use compared with the true-negative group included patients with true-positive results (odds ratio [OR], 1.60; 95% confidence interval [95% CI], 1.54-1.66), and positive groups with a colorectal cancer diagnosis (OR, 7.19; 95% CI, 6.12-8.44), polypectomy/no neoplasia (OR, 1.37; 95% CI, 1.27-1.48), and normal examination/no polypectomy (OR, 1.24; 95% CI, 1.18-1.30).
Conclusions: Given the large size of outreach programs, these small changes can cumulatively generate thousands of excess visits and have a substantial impact on total health care use. Therefore, these changes should be included in colorectal cancer screening cost models and their causes investigated further. Cancer 2017;123:3744-3753. © 2017 American Cancer Society.
Keywords: colorectal cancer; delivery of health care; early detection of cancer; primary health care.
© 2017 American Cancer Society.
Conflict of interest statement
Figures
References
-
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. - PubMed
-
- United States Preventive Services Task Force. AHRQ Publication 08-05124-EF-3. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Screening for colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement.
-
- Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–750. - PubMed
-
- Klabunde CN, Joseph DA, King JB, White A, Plescia M. Colorectal Cancer Screening Test Use - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(Early Release):1–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

