Human Induced Pluripotent Stem Cell-Derived Endothelial Cells for Three-Dimensional Microphysiological Systems
- PMID: 28622076
- PMCID: PMC5567879
- DOI: 10.1089/ten.TEC.2017.0133
Human Induced Pluripotent Stem Cell-Derived Endothelial Cells for Three-Dimensional Microphysiological Systems
Abstract
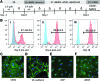
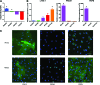
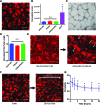
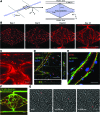
Microphysiological systems (MPS), or "organ-on-a-chip" platforms, aim to recapitulate in vivo physiology using small-scale in vitro tissue models of human physiology. While significant efforts have been made to create vascularized tissues, most reports utilize primary endothelial cells that hinder reproducibility. In this study, we report the use of human induced pluripotent stem cell-derived endothelial cells (iPS-ECs) in developing three-dimensional (3D) microvascular networks. We established a CDH5-mCherry reporter iPS cell line, which expresses the vascular endothelial (VE)-cadherin fused to mCherry. The iPS-ECs demonstrate physiological functions characteristic of primary endothelial cells in a series of in vitro assays, including permeability, response to shear stress, and the expression of endothelial markers (CD31, von Willibrand factor, and endothelial nitric oxide synthase). The iPS-ECs form stable, perfusable microvessels over the course of 14 days when cultured within 3D microfluidic devices. We also demonstrate that inhibition of TGF-β signaling improves vascular network formation by the iPS-ECs. We conclude that iPS-ECs can be a source of endothelial cells in MPS providing opportunities for human disease modeling and improving the reproducibility of 3D vascular networks.
Keywords: endothelial cells; induced pluripotent stem cells; microfluidics; vascularization.
Conflict of interest statement
S.C.G. is a cofounder of 4Design Biosciences, LLC. All other authors have no competing financial interests.
Figures
Similar articles
-
Stem cell-based vascularization of microphysiological systems.Stem Cell Reports. 2021 Sep 14;16(9):2058-2075. doi: 10.1016/j.stemcr.2021.03.015. Epub 2021 Apr 8. Stem Cell Reports. 2021. PMID: 33836144 Free PMC article. Review.
-
Induced Pluripotent Stem (iPS) Cell Culture Methods and Induction of Differentiation into Endothelial Cells.Methods Mol Biol. 2016;1357:311-27. doi: 10.1007/7651_2015_203. Methods Mol Biol. 2016. PMID: 25687301 Free PMC article.
-
Characterisation of human induced pluripotent stem cell-derived endothelial cells under shear stress using an easy-to-use microfluidic cell culture system.Biomed Microdevices. 2017 Oct 9;19(4):91. doi: 10.1007/s10544-017-0229-5. Biomed Microdevices. 2017. PMID: 28994005
-
Generation of functional endothelial cells with progenitor-like features from murine induced pluripotent stem cells.Vascul Pharmacol. 2016 Nov;86:94-108. doi: 10.1016/j.vph.2016.07.008. Epub 2016 Aug 25. Vascul Pharmacol. 2016. PMID: 27568462
-
Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling.J Mol Cell Cardiol. 2013 Sep;62:43-50. doi: 10.1016/j.yjmcc.2013.04.022. Epub 2013 Apr 30. J Mol Cell Cardiol. 2013. PMID: 23643470 Review.
Cited by
-
Stem cell-based vascularization of microphysiological systems.Stem Cell Reports. 2021 Sep 14;16(9):2058-2075. doi: 10.1016/j.stemcr.2021.03.015. Epub 2021 Apr 8. Stem Cell Reports. 2021. PMID: 33836144 Free PMC article. Review.
-
Recapitulating the Vasculature Using Organ-On-Chip Technology.Bioengineering (Basel). 2020 Feb 18;7(1):17. doi: 10.3390/bioengineering7010017. Bioengineering (Basel). 2020. PMID: 32085464 Free PMC article. Review.
-
Understanding angiogenesis and the role of angiogenic growth factors in the vascularisation of engineered tissues.Mol Biol Rep. 2021 Jan;48(1):941-950. doi: 10.1007/s11033-020-06108-9. Epub 2021 Jan 3. Mol Biol Rep. 2021. PMID: 33393005 Review.
-
A Hybrid Nanofiber/Paper Cell Culture Platform for Building a 3D Blood-brain Barrier Model.Small Methods. 2021 Sep 15;5(9):2100592. doi: 10.1002/smtd.202100592. Epub 2021 Aug 16. Small Methods. 2021. PMID: 34541301 Free PMC article.
-
Engineered Vasculature for Cancer Research and Regenerative Medicine.Micromachines (Basel). 2023 Apr 29;14(5):978. doi: 10.3390/mi14050978. Micromachines (Basel). 2023. PMID: 37241602 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

