Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation
- PMID: 28622359
- PMCID: PMC5473571
- DOI: 10.1371/journal.pone.0179648
Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation
Abstract
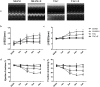
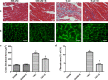
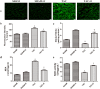
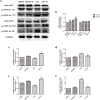
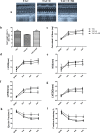
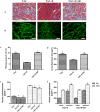
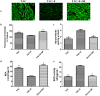
Aerobic exercise confers sustainable protection against cardiac hypertrophy and heart failure (HF). Nitric oxide synthase (NOS) and nitric oxide (NO) are known to play an important role in exercise-mediated cardioprotection, but the mechanism of NOS/NO stimulation during exercise remains unclear. The aim of this study is to determine the role of β3-adrenergic receptors (β3-ARs), NOS activation, and NO metabolites (nitrite and nitrosothiols) in the sustained cardioprotective effects of aerobic exercise. An HF model was constructed by transverse aortic constriction (TAC). Animals were treated with either moderate aerobic exercise by swimming for 9 weeks and/or the β3-AR-specific inhibitor SR59230A at 0.1 mg/kg/hour one day after TAC operation. Myocardial fibrosis, myocyte size, plasma catecholamine (CA) level, cardiac function and geometry were assessed using Masson's trichrome staining, FITC-labeled wheat germ agglutinin staining, enzyme-linked immuno sorbent assay (ELISA) and echocardiography, respectively. Western blot analysis was performed to elucidate the expression of target proteins. The concentration of myocardial NO production was evaluated using the nitrate reductase method. Myocardial oxidative stress was assessed by detecting the concentration of myocardial super oxidative dismutase (SOD), malonyldialdehyde (MDA), and reactive oxygen species (ROS). Aerobic exercise training improved dilated left ventricular function and partially attenuated the degree of cardiac hypertrophy and fibrosis in TAC mice. Moreover, the increased expression of β3-AR, activation of neuronal NOS (nNOS), and production of NO were detected after aerobic exercise training in TAC mice. However, selective inhibition of β3-AR by SR59230A abolished the upregulation and activation of nNOS induced NO production. Furthermore, aerobic exercise training decreased the myocardial ROS and MDA contents and increased myocardial levels of SOD; both effects were partially attenuated by SR59230A. Our study suggested that aerobic exercise training could improve cardiac systolic function and alleviate LV chamber dilation, cardiac fibrosis and hypertrophy in HF mice. The mechanism responsible for the protective effects of aerobic exercise is associated with the activation of the β3-AR-nNOS-NO pathway.
Conflict of interest statement
Figures


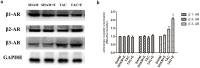

References
-
- Orso F, Fabbri G, Maggioni AP. Epidemiology of Heart Failure. Handb Exp Pharmacol. 2016. doi: 10.1007/164_2016_74 - DOI - PubMed
-
- Braunwald E. Heart failure. JACC Heart Fail. 2013;1: 1–20. doi: 10.1016/j.jchf.2012.10.002 - DOI - PubMed
-
- Roof SR, Tang L, Ostler JE, Periasamy M, Gyorke S, Billman GE, et al. Neuronal nitric oxide synthase is indispensable for the cardiac adaptive effects of exercise. Basic Res Cardiol. 2013;108: 332 doi: 10.1007/s00395-013-0332-6 - DOI - PMC - PubMed
-
- Piepoli MF, Davos C, Francis DP, Coats AJ, ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328: 189 doi: 10.1136/bmj.37938.645220.EE - DOI - PMC - PubMed
-
- Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005;569: 913–924. doi: 10.1113/jphysiol.2005.095729 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

