AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance
- PMID: 28622524
- PMCID: PMC5553560
- DOI: 10.1016/j.molcel.2017.05.032
AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance
Abstract
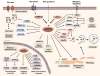
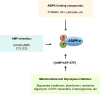
AMPK is a highly conserved master regulator of metabolism, which restores energy balance during metabolic stress both at the cellular and physiological levels. The identification of numerous AMPK targets has helped explain how AMPK restores energy homeostasis. Recent advancements illustrate novel mechanisms of AMPK regulation, including changes in subcellular localization and phosphorylation by non-canonical upstream kinases. Notably, the therapeutic potential of AMPK is widely recognized and heavily pursued for treatment of metabolic diseases such as diabetes, but also obesity, inflammation, and cancer. Moreover, the recently solved crystal structure of AMPK has shed light both into how nucleotides activate AMPK and, importantly, also into the sites bound by small molecule activators, thus providing a path for improved drugs.
Keywords: AMPK; energy restoration; metabolic adaptation; therapeutic activation; trimeric complex.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Asby DJ, Cuda F, Beyaert M, Houghton FD, Cagampang FR, Tavassoli A. AMPK Activation via Modulation of De Novo Purine Biosynthesis with an Inhibitor of ATIC Homodimerization. Chem Biol. 2015;22:838–848. - PubMed
-
- Calabrese MF, Rajamohan F, Harris MS, Caspers NL, Magyar R, Withka JM, Wang H, Borzilleri KA, Sahasrabudhe PV, Hoth LR, et al. Structural Basis for AMPK Activation: Natural and Synthetic Ligands Regulate Kinase Activity from Opposite Poles by Different Molecular Mechanisms. Structure/Folding and Design. 2014;22:1161–1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

