Antimycobacterial Metabolism: Illuminating Mycobacterium tuberculosis Biology and Drug Discovery
- PMID: 28622844
- PMCID: PMC5564221
- DOI: 10.1016/j.tim.2017.05.007
Antimycobacterial Metabolism: Illuminating Mycobacterium tuberculosis Biology and Drug Discovery
Abstract
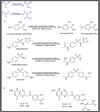
Bacteria are capable of performing a number of biotransformations that may activate or deactivate xenobiotics. Recent efforts have utilized metabolomics techniques to study the fate of small-molecule antibacterials within the targeted organism. Examples involving Mycobacterium tuberculosis are reviewed and analyzed with regard to the insights they provide as to both activation and deactivation of the antibacterial. The studies, in particular, shed light on biosynthetic transformations performed by M. tuberculosis while suggesting avenues for the evolution of chemical tools, highlighting potential areas for drug discovery, and mechanisms of approved drugs. A two-pronged approach investigating the metabolism of antibacterials within both the host and bacterium is outlined and will be of value to both the chemical biology and drug discovery fields.
Keywords: antibacterial; biotransformation; metabolism; metabolomics; small molecule; tuberculosis.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures





References
-
- Zumla A, et al. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov. 2013;12:388–404. - PubMed
-
- Sassetti CM, et al. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. - PubMed
-
- Zhang Y, et al. The catalase–peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. - PubMed
-
- Banerjee A, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

