Sialic acid linkage differentiation of glycopeptides using capillary electrophoresis - electrospray ionization - mass spectrometry
- PMID: 28623326
- PMCID: PMC5473812
- DOI: 10.1038/s41598-017-03838-y
Sialic acid linkage differentiation of glycopeptides using capillary electrophoresis - electrospray ionization - mass spectrometry
Abstract
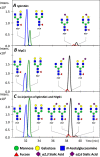
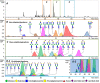
Sialylation is a glycosylation feature that occurs in different linkages at the non-reducing end of a glycan moiety, the linkage isomers are often differentially associated with various biological processes. Due to very similar physico-chemical properties, the separation of isomeric sialylated glycopeptides remains challenging but of utmost importance in the biomedicine and biotechnology, including biomarker discovery, glyco-engineering and biopharmaceutical characterization. This study presents the implementation of a high-resolution separation platform based on capillary electrophoresis - mass spectrometry (CE-MS) allowing for the selective analysis of α2,3- and α2,6-sialylated glycopeptides. These differentially linked glycopeptides showed an identical fragmentation pattern (collision induced dissociation) but different electrophoretic mobilities, allowing for baseline separation of the different linkages without the need for an extensive sample preparation. The different migration behavior between the two moieties was found to correlate with differences in pKa values. Using a novel methodology adapted from the so-called internal standard CE approach, a relative difference of 3.4·10-2 in pKa unit was determined. This approach was applied for the analysis of tryptic glycopeptides of prostate specific antigen, which shows highly complex and heterogeneous glycosylation. The developed platform therefore appears attractive for the identification of differentially linked sialic acids that may be related to pathological conditions.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Taylor, M. E. & Drickamer, K. Introduction to glycobiology. 283 (Oxford University Press, 2011).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

