EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies
- PMID: 28623376
- PMCID: PMC5541084
- DOI: 10.1007/s00259-017-3740-2
EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies
Abstract
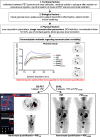
Quantitative positron emission tomography/computed tomography (PET/CT) can be used as diagnostic or prognostic tools (i.e. single measurement) or for therapy monitoring (i.e. longitudinal studies) in multicentre studies. Use of quantitative parameters, such as standardized uptake values (SUVs), metabolic active tumor volumes (MATVs) or total lesion glycolysis (TLG), in a multicenter setting requires that these parameters be comparable among patients and sites, regardless of the PET/CT system used. This review describes the motivations and the methodologies for quantitative PET/CT performance harmonization with emphasis on the EANM Research Ltd. (EARL) Fluorodeoxyglucose (FDG) PET/CT accreditation program, one of the international harmonization programs aiming at using FDG PET as a quantitative imaging biomarker. In addition, future accreditation initiatives will be discussed. The validation of the EARL accreditation program to harmonize SUVs and MATVs is described in a wide range of tumor types, with focus on therapy assessment using either the European Organization for Research and Treatment of Cancer (EORTC) criteria or PET Evaluation Response Criteria in Solid Tumors (PERCIST), as well as liver-based scales such as the Deauville score. Finally, also presented in this paper are the results from a survey across 51 EARL-accredited centers reporting how the program was implemented and its impact on daily routine and in clinical trials, harmonization of new metrics such as MATV and heterogeneity features.
Keywords: Deauville score; EARL accreditation; EORTC; Harmonization; MATV; PERCIST; PET/CT; SUV.
Conflict of interest statement
The authors do not have any financial conflict of interest to disclose.
Professor Aide received a research grant from Siemens R&D for research described in the present review (reference 42).
Figures



References
-
- Devriese J, Beels L, Maes A, Van De Wiele C, Gheysens O, Pottel H. Review of clinically accessible methods to determine lean body mass for normalization of standardized uptake values. The quarterly journal of nuclear medicine and molecular imaging: official publication of the Italian Association of Nuclear Medicine (AIMN) and the International Association of Radiopharmacology (IAR), and Section of the So. 2016;60:1–11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

