Development of a prototype immunochromatographic test for rapid diagnosis of respiratory adenovirus infection
- PMID: 28623675
- PMCID: PMC9425546
- DOI: 10.1016/j.bjid.2017.03.023
Development of a prototype immunochromatographic test for rapid diagnosis of respiratory adenovirus infection
Abstract
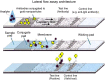
Human adenoviruses comprise an important group of etiologic agents that are responsible for various diseases in adults and children, such as respiratory, ocular, gastroenteric, and urinary infections. In immunocompromised and organ-transplanted individuals, these agents can cause generalized infections. Rapid diagnostic methods for detecting these infectious agents are not widely available. The aim of this work was to produce monoclonal and polyclonal anti-adenovirus antibodies to be used in a rapid diagnostic test for respiratory infections. Adenovirus hexons were satisfactorily purified by ultracentrifugation and chromatography. After virus purification, anti-hexon monoclonal antibodies were produced and characterized, following classical methods. Antibodies were specific for adenoviruses 2, 3, 5, and 41. The proposed immunochromatographic test was standardized using colloidal gold. The standardization of the rapid test was sufficient to detect adenovirus antigens (in nasopharyngeal lavage samples) with sensitivity of 100% and specificity of 85% when compared to direct immunofluorescence. The immunochromatographic assay prototype was sufficiently sensitive to detect B (3), C (2 and 5), and F (41) adenovirus samples. Although based on preliminary data, the test demonstrated the same performance as direct immunofluorescence, but with the advantage of being a point-of-care test. Further studies are still needed to confirm its effectiveness in clinical practice.
Keywords: Adenovirus; Immunochromatographic test; Monoclonal antibodies.
Copyright © 2017 Sociedade Brasileira de Infectologia. Published by Elsevier Editora Ltda. All rights reserved.
Figures

Similar articles
-
Performance of a new immunochromatographic assay for detection of adenoviruses in children.J Clin Virol. 2009 Feb;44(2):173-5. doi: 10.1016/j.jcv.2008.11.002. Epub 2008 Dec 19. J Clin Virol. 2009. PMID: 19101199
-
[New monoclonal kits for the diagnosis of the adenoviral infection].Vopr Virusol. 2014;59(5):43-6. Vopr Virusol. 2014. PMID: 25895211 Russian.
-
Rapid detection and serotyping of adenovirus by direct immunofluorescence.J Med Virol. 1997 Mar;51(3):198-201. doi: 10.1002/(sici)1096-9071(199703)51:3<198::aid-jmv9>3.0.co;2-1. J Med Virol. 1997. PMID: 9139083
-
Adenoviruses in immunocompromised hosts.Clin Microbiol Rev. 2008 Oct;21(4):704-15. doi: 10.1128/CMR.00052-07. Clin Microbiol Rev. 2008. PMID: 18854488 Free PMC article. Review.
-
Adenovirus infections in immunocompetent and immunocompromised patients.Clin Microbiol Rev. 2014 Jul;27(3):441-62. doi: 10.1128/CMR.00116-13. Clin Microbiol Rev. 2014. PMID: 24982316 Free PMC article. Review.
Cited by
-
Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG.Am J Transl Res. 2020 Apr 15;12(4):1348-1354. eCollection 2020. Am J Transl Res. 2020. PMID: 32355546 Free PMC article.
-
Different Clinical Manifestations of Adenoviral Infection Confirmed Using Point-of-Care Testing in a Group of Hospitalized Children.Pediatr Rep. 2022 Dec 22;15(1):1-8. doi: 10.3390/pediatric15010001. Pediatr Rep. 2022. PMID: 36649001 Free PMC article. Review.
-
Simultaneous serodetection of major rat infectious pathogens by a multiplex immunochromatographic assay.Exp Anim. 2021 May 13;70(2):161-168. doi: 10.1538/expanim.20-0099. Epub 2020 Nov 12. Exp Anim. 2021. PMID: 33177250 Free PMC article.
-
Unleashing the power of colloidal gold immunochromatographic assays for plant virus diagnostics.MethodsX. 2023 Nov 29;12:102498. doi: 10.1016/j.mex.2023.102498. eCollection 2024 Jun. MethodsX. 2023. PMID: 38089155 Free PMC article. Review.
-
Multiplex Immunochromatographic Assay for Serologic Diagnosis of Major Infectious Diseases in Laboratory Mice.J Am Assoc Lab Anim Sci. 2019 Nov 1;58(6):790-795. doi: 10.30802/AALAS-JAALAS-19-000008. Epub 2019 Sep 13. J Am Assoc Lab Anim Sci. 2019. PMID: 31519225 Free PMC article.
References
-
- Belfort R., Barbosa J.B., Junior, Regatieri C.V.S., et al. Diagnóstico de conjuntivite adenoviral pelo RPS Adenodetector (2007) Arq Bras Oftalmol. 2007;70:441–444. - PubMed
-
- Ferone E.A., Berezin E.N., Durigon G.S., et al. Clinical and epidemiological aspects related to the detection of adenovirus or respiratory syncytial virus in infants hospitalized for acute lower respiratory tract infection. J Pediatr (Rio J) 2014;90:42–49. - PubMed
-
- Levent F., Greer J.M., Snider M., Demmeler-Harrison G.J. Performance of a new immunochromatographic assay for detection of adenoviruses in children. J Clin Virol. 2009;44:173–175. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

