Evaluation of Antisense Oligonucleotides Targeting ATXN3 in SCA3 Mouse Models
- PMID: 28624196
- PMCID: PMC5415970
- DOI: 10.1016/j.omtn.2017.04.005
Evaluation of Antisense Oligonucleotides Targeting ATXN3 in SCA3 Mouse Models
Abstract
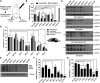
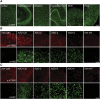
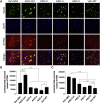
The most common dominantly inherited ataxia, spinocerebellar ataxia type 3 (SCA3), is an incurable neurodegenerative disorder caused by a CAG repeat expansion in the ATXN3 gene that encodes an abnormally long polyglutamine tract in the disease protein, ATXN3. Mice lacking ATXN3 are phenotypically normal; hence, disease gene suppression offers a compelling approach to slow the neurodegenerative cascade in SCA3. Here we tested antisense oligonucleotides (ASOs) that target human ATXN3 in two complementary mouse models of SCA3: yeast artificial chromosome (YAC) MJD-Q84.2 (Q84) mice expressing the full-length human ATXN3 gene and cytomegalovirus (CMV) MJD-Q135 (Q135) mice expressing a human ATXN3 cDNA. Intracerebroventricular injection of ASOs resulted in widespread delivery to the most vulnerable brain regions in SCA3. In treated Q84 mice, three of five tested ASOs reduced disease protein levels by >50% in the diencephalon, cerebellum, and cervical spinal cord. Two ASOs also significantly reduced mutant ATXN3 in the mouse forebrain and resulted in no signs of astrogliosis or microgliosis. In Q135 mice expressing a single ATXN3 isoform via a cDNA transgene, ASOs did not result in similar robust ATXN3 silencing. Our results indicate that ASOs targeting full-length human ATXN3 would likely be well tolerated and could lead to a preventative therapy for SCA3.
Keywords: ASO; ATXN3; MJD; Machado-Joseph disease; SCA3; antisense oligonucleotide; polyglutamine disease; spinocerebellar ataxia type 3.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Alves S., Nascimento-Ferreira I., Auregan G., Hassig R., Dufour N., Brouillet E., Pedroso de Lima M.C., Hantraye P., Pereira de Almeida L., Déglon N. Allele-specific RNA silencing of mutant ataxin-3 mediates neuroprotection in a rat model of Machado-Joseph disease. PLoS ONE. 2008;3:e3341. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

