Statin Trials, Cardiovascular Events, and Coronary Artery Calcification: Implications for a Trial-Based Approach to Statin Therapy in MESA
- PMID: 28624395
- PMCID: PMC5723240
- DOI: 10.1016/j.jcmg.2017.01.029
Statin Trials, Cardiovascular Events, and Coronary Artery Calcification: Implications for a Trial-Based Approach to Statin Therapy in MESA
Abstract
Objectives: This study sought to determine whether coronary artery calcium (CAC) could be used to optimize statin allocation among individuals for whom trial-based evidence supports efficacy of statin therapy.
Background: Recently, allocation of statins was proposed for primary prevention of atherosclerotic cardiovascular disease (ASCVD) based on proven efficacy from randomized controlled trials (RCTs) of statin therapy, a so-called trial-based approach.
Methods: The study used data from MESA (Multi-Ethnic Study of Atherosclerosis) with 5,600 men and women, 45 to 84 years of age, and free of clinical ASCVD, lipid-lowering therapy, or missing information for risk factors at baseline examination.
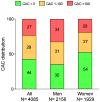
Results: During 10 years' follow-up, 354 ASCVD and 219 hard coronary heart disease (CHD) events occurred. Based on enrollment criteria for 7 RCTs of statin therapy in primary prevention, 73% of MESA participants (91% of those >55 years of age) were eligible for statin therapy according to a trial-based approach. Among those individuals, CAC = 0 was common (44%) and was associated with low rates of ASCVD and CHD (3.9 and 1.7, respectively, per 1,000 person-years). There was a graded increase in event rates with increasing CAC score, and in individuals with CAC >100 (27% of participants) the rates of ASCVD and CHD were 18.9 and 12.7, respectively. Consequently, the estimated number needed to treat (NNT) in 10 years to prevent 1 event varied greatly according to CAC score. For ASCVD events, the NNT was 87 for CAC = 0 and 19 for CAC >100. For CHD events, the NNT was 197 for CAC = 0 and 28 for CAC >100.
Conclusions: Most MESA participants qualified for trial-based primary prevention with statins. Among the individuals for whom trial-based evidence supports efficacy of statin therapy, CAC = 0 and CAC >100 were common and associated with low and high cardiovascular risks, respectively. This information may guide shared decision making aimed at targeting evidence-based statins to those who are likely to benefit the most.
Keywords: cardiovascular disease; guideline; lipoproteins; primary prevention; statin.
Copyright © 2018 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

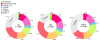


Comment in
-
Using Trial Eligibility to Personalize Statin Therapy Appears No More Accurate Than a Coin Flip in Determining High-Risk Status.JACC Cardiovasc Imaging. 2018 Feb;11(2 Pt 1):231-233. doi: 10.1016/j.jcmg.2017.02.019. Epub 2017 Jun 14. JACC Cardiovasc Imaging. 2018. PMID: 28624410 No abstract available.
References
-
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2889–2934. - PubMed
-
- JBS3 Board. Joint British Societies' consensus recommendations for the prevention of cardiovascular disease (JBS3) Heart. 2014;100(Suppl 2):ii1–ii67. - PubMed
-
- Ridker PM, Wilson PWF. A trial-based approach to statin guidelines. JAMA. 2013;310:1123–1124. - PubMed
-
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- N01 HC095167/HC/NHLBI NIH HHS/United States
- N01 HC095161/HC/NHLBI NIH HHS/United States
- N01 HC095164/HC/NHLBI NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- N01 HC095169/HC/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- N01 HC095168/HC/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- N01 HC095166/HC/NHLBI NIH HHS/United States
- N01 HC095160/HC/NHLBI NIH HHS/United States
- N01 HC095165/HC/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- N01 HC095163/HC/NHLBI NIH HHS/United States
- N01 HC095162/HC/NHLBI NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
- N01 HC095159/HC/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

