Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients
- PMID: 28624961
- PMCID: PMC5563341
- DOI: 10.1007/s00401-017-1744-4
Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients
Abstract
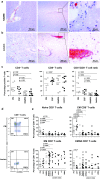
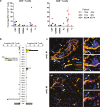
T cells are considered pivotal in the pathology of multiple sclerosis (MS), but their function and antigen specificity are unknown. To unravel the role of T cells in MS pathology, we performed a comprehensive analysis on T cells recovered from paired blood, cerebrospinal fluid (CSF), normal-appearing white matter (NAWM) and white matter lesions (WML) from 27 MS patients with advanced disease shortly after death. The differentiation status of T cells in these compartments was determined by ex vivo flow cytometry and immunohistochemistry. T-cell reactivity in short-term T-cell lines (TCL), generated by non-specific stimulation of T cells recovered from the same compartments, was determined by intracellular cytokine flow cytometry. Central memory T cells predominated in CSF and effector memory T cells were enriched in NAWM and WML. WML-derived CD8+ T cells represent chronically activated T cells expressing a cytotoxic effector phenotype (CD95L and granzyme B) indicative for local antigenic stimulation (CD137). The same lesions also contained higher CD8+ T-cell frequencies expressing co-inhibitory (TIM3 and PD1) and co-stimulatory (ICOS) T-cell receptors, yet no evidence for T-cell senescence (CD57) was observed. The oligoclonal T-cell receptor (TCR) repertoire, particularly among CD8+ T cells, correlated between TCL generated from anatomically separated WML of the same MS patient, but not between paired NAWM and WML. Whereas no substantial T-cell reactivity was detected towards seven candidate human MS-associated autoantigens (cMSAg), brisk CD8+ T-cell reactivity was detected in multiple WML-derived TCL towards autologous Epstein-Barr virus (EBV) infected B cells (autoBLCL). In one MS patient, the T-cell response towards autoBLCL in paired intra-lesional TCL was dominated by TCRVβ2+CD8+ T cells, which were localized in the parenchyma of the respective tissues expressing a polarized TCR and CD8 expression suggesting immunological synapse formation in situ. Collectively, the data suggest the involvement of effector memory cytotoxic T cells recognizing antigens expressed by autoBLCL, but not the assayed human cMSAg, in WML of MS patients.
Keywords: Autoantigens; CD8 T cells; Epstein–Barr virus; Multiple sclerosis; Pathogenesis.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures





References
-
- Angelini DF, Serafini B, Piras E, Severa M, Coccia EM, Rosicarelli B, Ruggieri S, Gasperini C, Buttari F, Centonze D, Mechelli R, Salvetti M, Borsellino G, Aloisi F, Battistini L. Increased CD8+ T cell response to Epstein–Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013;9:e1003220. doi: 10.1371/journal.ppat.1003220. - DOI - PMC - PubMed
-
- Annibali V, Ristori G, Angelini DF, Serafini B, Mechelli R, Cannoni S, Romano S, Paolillo A, Abderrahim H, Diamantini A, Borsellino G, Aloisi F, Battistini L, Salvetti M. CD161highCD8+ T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134:542–554. doi: 10.1093/brain/awq354. - DOI - PubMed
-
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schröder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

