Semi-quantitative models for identifying potent and selective transthyretin amyloidogenesis inhibitors
- PMID: 28625364
- PMCID: PMC5557047
- DOI: 10.1016/j.bmcl.2017.05.080
Semi-quantitative models for identifying potent and selective transthyretin amyloidogenesis inhibitors
Abstract
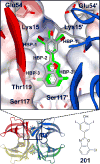
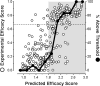
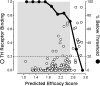
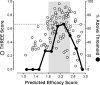
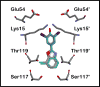
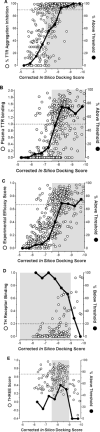
Rate-limiting dissociation of the tetrameric protein transthyretin (TTR), followed by monomer misfolding and misassembly, appears to cause degenerative diseases in humans known as the transthyretin amyloidoses, based on human genetic, biochemical and pharmacologic evidence. Small molecules that bind to the generally unoccupied thyroxine binding pockets in the native TTR tetramer kinetically stabilize the tetramer, slowing subunit dissociation proportional to the extent that the molecules stabilize the native state over the dissociative transition state-thereby inhibiting amyloidogenesis. Herein, we use previously reported structure-activity relationship data to develop two semi-quantitative algorithms for identifying the structures of potent and selective transthyretin kinetic stabilizers/amyloidogenesis inhibitors. The viability of these prediction algorithms, in particular the more robust in silico docking model, is perhaps best validated by the clinical success of tafamidis, the first-in-class drug approved in Europe, Japan, South America, and elsewhere for treating transthyretin aggregation-associated familial amyloid polyneuropathy. Tafamidis is also being evaluated in a fully-enrolled placebo-controlled clinical trial for its efficacy against TTR cardiomyopathy. These prediction algorithms will be useful for identifying second generation TTR kinetic stabilizers, should these be needed to ameliorate the central nervous system or ophthalmologic pathology caused by TTR aggregation in organs not accessed by oral tafamidis administration.
Keywords: Amyloid; Familial amyloid polyneuropathy; In silico docking; Inhibitor; Prediction algorithms; Senile systemic amyloidosis; Structural biology; Structure-based drug design; TTR; Thyroid hormone receptors; Transthyretin.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Personalized medicine approach for optimizing the dose of tafamidis to potentially ameliorate wild-type transthyretin amyloidosis (cardiomyopathy).Amyloid. 2015;22(3):175-80. doi: 10.3109/13506129.2015.1063485. Epub 2015 Jul 25. Amyloid. 2015. PMID: 26193961 Free PMC article.
-
Synthesis and characterization of potent bivalent amyloidosis inhibitors that bind prior to transthyretin tetramerization.J Am Chem Soc. 2003 Nov 5;125(44):13404-14. doi: 10.1021/ja030294z. J Am Chem Soc. 2003. PMID: 14583036
-
Genistein, a natural product from soy, is a potent inhibitor of transthyretin amyloidosis.Proc Natl Acad Sci U S A. 2005 Oct 11;102(41):14545-50. doi: 10.1073/pnas.0501609102. Epub 2005 Sep 29. Proc Natl Acad Sci U S A. 2005. PMID: 16195386 Free PMC article.
-
Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses.Acc Chem Res. 2005 Dec;38(12):911-21. doi: 10.1021/ar020073i. Acc Chem Res. 2005. PMID: 16359163 Review.
-
Structure-based design of kinetic stabilizers that ameliorate the transthyretin amyloidoses.Curr Opin Struct Biol. 2010 Feb;20(1):54-62. doi: 10.1016/j.sbi.2009.12.009. Epub 2010 Feb 3. Curr Opin Struct Biol. 2010. PMID: 20133122 Free PMC article. Review.
Cited by
-
Hydroxybiphenylamide GroEL/ES Inhibitors Are Potent Antibacterials against Planktonic and Biofilm Forms of Staphylococcus aureus.J Med Chem. 2018 Dec 13;61(23):10651-10664. doi: 10.1021/acs.jmedchem.8b01293. Epub 2018 Nov 15. J Med Chem. 2018. PMID: 30392371 Free PMC article.
-
A designed protein binding-pocket to control excited-state intramolecular proton transfer fluorescence.Org Biomol Chem. 2019 Jan 31;17(5):1076-1080. doi: 10.1039/c8ob02673d. Org Biomol Chem. 2019. PMID: 30534794 Free PMC article.
-
Sulfonamido-2-arylbenzoxazole GroEL/ES Inhibitors as Potent Antibacterials against Methicillin-Resistant Staphylococcus aureus (MRSA).J Med Chem. 2018 Aug 23;61(16):7345-7357. doi: 10.1021/acs.jmedchem.8b00989. Epub 2018 Aug 14. J Med Chem. 2018. PMID: 30060666 Free PMC article.
-
Review on the Structures and Activities of Transthyretin Amyloidogenesis Inhibitors.Drug Des Devel Ther. 2020 Mar 10;14:1057-1081. doi: 10.2147/DDDT.S237252. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32210536 Free PMC article. Review.
-
Proposing a minimal set of metrics and methods to predict probabilities of amyloidosis disease and onset age in individuals.Aging (Albany NY). 2020 Nov 18;12(22):22356-22369. doi: 10.18632/aging.202208. Epub 2020 Nov 18. Aging (Albany NY). 2020. PMID: 33203794 Free PMC article.
References
-
- Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004;16:67–75. - PubMed
-
- Buxbaum JN, Tagoe CE. The genetics of the amyloidoses. Annu Rev Med. 2000;51:543–69. - PubMed
-
- Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, Buxbaum JN. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–73. - PubMed
-
- Gambetti P, Russo C. Human brain amyloidoses. Nephrol Dial Transplant. 1998;13(Suppl 7):33–40. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

