Heterogeneous Ribosomes Preferentially Translate Distinct Subpools of mRNAs Genome-wide
- PMID: 28625553
- PMCID: PMC5548184
- DOI: 10.1016/j.molcel.2017.05.021
Heterogeneous Ribosomes Preferentially Translate Distinct Subpools of mRNAs Genome-wide
Abstract
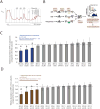
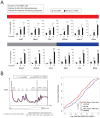
Emerging studies have linked the ribosome to more selective control of gene regulation. However, an outstanding question is whether ribosome heterogeneity at the level of core ribosomal proteins (RPs) exists and enables ribosomes to preferentially translate specific mRNAs genome-wide. Here, we measured the absolute abundance of RPs in translating ribosomes and profiled transcripts that are enriched or depleted from select subsets of ribosomes within embryonic stem cells. We find that heterogeneity in RP composition endows ribosomes with differential selectivity for translating subpools of transcripts, including those controlling metabolism, cell cycle, and development. As an example, mRNAs enriched in binding to RPL10A/uL1-containing ribosomes are shown to require RPL10A/uL1 for their efficient translation. Within several of these transcripts, this level of regulation is mediated, at least in part, by internal ribosome entry sites. Together, these results reveal a critical functional link between ribosome heterogeneity and the post-transcriptional circuitry of gene expression.
Keywords: 5′ UTR regulatory elements; IRES elements; SRM; gene regulation; internal ribosome entry site elements; ribosome heterogeneity; selected reaction monitoring; translational control.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Comment in
-
A division of labor in cells' protein factories.Science. 2017 Jun 23;356(6344):1218-1219. doi: 10.1126/science.356.6344.1218. Science. 2017. PMID: 28642386 No abstract available.
References
-
- Anger AM, Armache JP, Berninghausen O, Habeck M, Subklewe M, Wilson DN, Beckmann R. Structures of the human and Drosophila 80S ribosome. Nature. 2013;497:80–85. - PubMed
-
- Boehringer D, Thermann R, Ostareck-Lederer A, Lewis JD, Stark H. Structure of the hepatitis C virus IRES bound to the human 80S ribosome: remodeling of the HCV IRES. Structure. 2005;13:1695–1706. - PubMed
-
- Carter MS, Sarnow P. Distinct mRNAs that encode La autoantigen are differentially expressed and contain internal ribosome entry sites. J Biol Chem. 2000;275:28301–28307. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

