The CEP19-RABL2 GTPase Complex Binds IFT-B to Initiate Intraflagellar Transport at the Ciliary Base
- PMID: 28625565
- PMCID: PMC5556974
- DOI: 10.1016/j.devcel.2017.05.016
The CEP19-RABL2 GTPase Complex Binds IFT-B to Initiate Intraflagellar Transport at the Ciliary Base
Abstract
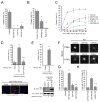
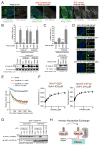
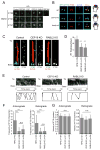
Highly conserved intraflagellar transport (IFT) protein complexes direct both the assembly of primary cilia and the trafficking of signaling molecules. IFT complexes initially accumulate at the base of the cilium and periodically enter the cilium, suggesting an as-yet-unidentified mechanism that triggers ciliary entry of IFT complexes. Using affinity-purification and mass spectrometry of interactors of the centrosomal and ciliopathy protein, CEP19, we identify CEP350, FOP, and the RABL2B GTPase as proteins organizing the first known mechanism directing ciliary entry of IFT complexes. We discover that CEP19 is recruited to the ciliary base by the centriolar CEP350/FOP complex and then specifically captures GTP-bound RABL2B, which is activated via its intrinsic nucleotide exchange. Activated RABL2B then captures and releases its single effector, the intraflagellar transport B holocomplex, from the large pool of pre-docked IFT-B complexes, and thus initiates ciliary entry of IFT.
Keywords: CEP19; CEP350; FGFR1OP; RABL2; cilia; ciliopathy; intraflagellar transport; obesity; retinal degeneration; small GTPase.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Gating Ciliary Transport.Dev Cell. 2017 Jul 10;42(1):5-6. doi: 10.1016/j.devcel.2017.06.016. Dev Cell. 2017. PMID: 28697332
References
-
- Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. - PubMed
-
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

