Structure and Function of Viral Deubiquitinating Enzymes
- PMID: 28625850
- PMCID: PMC7094624
- DOI: 10.1016/j.jmb.2017.06.010
Structure and Function of Viral Deubiquitinating Enzymes
Abstract
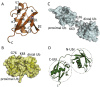
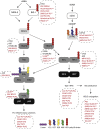
Post-translational modification of cellular proteins by ubiquitin regulates numerous cellular processes, including innate and adaptive immune responses. Ubiquitin-mediated control over these processes can be reversed by cellular deubiquitinating enzymes (DUBs), which remove ubiquitin from cellular targets and depolymerize polyubiquitin chains. The importance of protein ubiquitination to host immunity has been underscored by the discovery of viruses that encode proteases with deubiquitinating activity, many of which have been demonstrated to actively corrupt cellular ubiquitin-dependent processes to suppress innate antiviral responses and promote viral replication. DUBs have now been identified in diverse viral lineages, and their characterization is providing valuable insights into virus biology and the role of the ubiquitin system in host antiviral mechanisms. Here, we provide an overview of the structural biology of these fascinating viral enzymes and their role innate immune evasion and viral replication.
Keywords: innate immune evasion; papain-like protease; ubiquitin; viral deubiquitinating enzyme; viruses.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




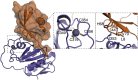
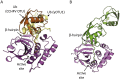

References
-
- Komander D., Rape M. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. - PubMed
-
- Husnjak K., Dikic I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 2012;81:291–322. - PubMed
-
- Akutsu M., Dikic I., Bremm A. Ubiquitin chain diversity at a glance. J. Cell Sci. 2016;129:875–880. - PubMed
-
- Kulathu Y., Komander D. Atypical ubiquitylation—the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 2012;13:508–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

