Perpetual change: autophagy, the endothelium, and response to vascular injury
- PMID: 28626046
- PMCID: PMC6608075
- DOI: 10.1189/jlb.3RU1116-484RR
Perpetual change: autophagy, the endothelium, and response to vascular injury
Abstract
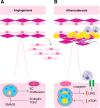
Current studies of vascular health, aging, and autophagy emphasize how the endothelium adapts to stress and contributes to disease. The endothelium is far from an inert barrier to blood-borne cells, pathogens, and chemical signals; rather, it actively translates circulating mediators into tissue responses, changing rapidly in response to physiologic stressors. Macroautophagy-the cellular ingestion of effete organelles and protein aggregates to provide anabolic substrates to fuel bioenergetics in times of stress-plays an important role in endothelial cell homeostasis, vascular remodeling, and disease. These roles include regulating vascular tone, sustaining or limiting cell survival, and contributing to the development of atherosclerosis secondary to infection, inflammation, and angiogenesis. Autophagy modulates these critical functions of the endothelium in a dynamic and perpetual response to tissue and intravascular cues.
Keywords: ATG; HMGB1; atherosclerosis; cardiovascular.
© Society for Leukocyte Biology.
Figures



References
-
- Shirakabe, A. , Zhai, P. , Ikeda, Y. , Saito, T. , Maejima, Y. , Hsu, C.‐P. , Nomura, M. , Egashira, K. , Levine, B. , Sadoshima, J. (2016) Drp1‐dependent mitochondrial autophagy plays a protective role against pressure overload‐induced mitochondrial dysfunction and heart failure. Circulation 133, 1249–1263. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

