Increased vascular and uteroplacental matrix metalloproteinase-1 and -7 levels and collagen type I deposition in hypertension in pregnancy: role of TNF-α
- PMID: 28626073
- PMCID: PMC5625170
- DOI: 10.1152/ajpheart.00207.2017
Increased vascular and uteroplacental matrix metalloproteinase-1 and -7 levels and collagen type I deposition in hypertension in pregnancy: role of TNF-α
Abstract
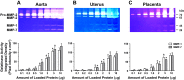
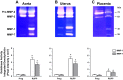
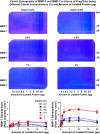
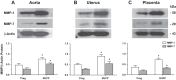
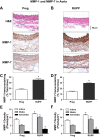
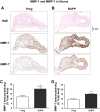
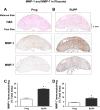
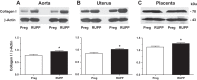
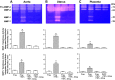
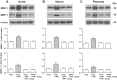
Preeclampsia is a pregnancy-related disorder manifested as maternal hypertension in pregnancy (HTN-Preg) and fetal growth restriction. Placental ischemia could be an initiating event that leads to abnormal vascular and uteroplacental remodeling in HTN-Preg; however, the molecular targets and intermediary mechanisms involved are unclear. We tested the hypothesis that placental ischemia could target vascular and uteroplacental matrix metalloproteinases (MMPs) through an inflammatory cytokine-mediated mechanism. MMP levels and distribution were measured in the aorta, uterus, and placenta of normal pregnant (Preg) rats and pregnant rats with reduced uterine perfusion pressure (RUPP). Maternal blood pressure was higher and the litter size and pup weight were lower in RUPP compared with Preg rats. Gelatin zymography showed prominent uterine MMP-2 and MMP-9 activity that was dependent on the amount of loaded protein. At saturating protein loading, both gelatin and casein zymography revealed two additional bands corresponding to MMP-1 and MMP-7 that were greater in the aorta, uterus, and placenta of RUPP compared with Preg rats. Western blots and immunohistochemistry confirmed increased MMP-1 and MMP-7 in the aorta, uterus, and placenta of RUPP versus Preg rats. The levels of MMP-1 and MMP-7 substrate collagen type I were greater in tissues of RUPP compared with Preg rats. In organ culture, TNF-α increased MMP-1 and MMP-7 in the aorta, uterus, and placenta of Preg rats, and a TNF-α antagonist prevented the increases in MMPs in tissues of RUPP rats. Thus, placental ischemia, possibly through TNF-α, increases vascular and uteroplacental MMP-1 and MMP-7, which, in turn, alter collagen deposition and cause inadequate tissue remodeling in HTN-Preg. Cytokine antagonists may reverse the increase in MMP-1 and MMP-7 expression/activity and, in turn, restore proper vascular and uteroplacental remodeling in HTN-Preg and preeclampsia.NEW & NOTEWORTHY The molecular mechanisms of preeclampsia are unclear, making it difficult to predict, prevent, or manage the pregnancy-associated disorder. This study showed that placental ischemia, possibly through the release of TNF-α, causes increases in the levels of matrix metalloproteinase (MMP)-1 and MMP-7, which could alter collagen deposition and cause inadequate uteroplacental and vascular remodeling in hypertension in pregnancy. The data suggest that targeting MMP-1 and MMP-7 and their upstream modulators, such as TNF-α, could provide a new approach in the management of hypertension in pregnancy and preeclampsia.
Keywords: aorta; placenta; preeclampsia; remodeling; tumor necrosis factor-α; uterus.
Copyright © 2017 the American Physiological Society.
Figures

Comment in
-
Uncovering novel roles for matrix metalloproteinases in preeclampsia.Am J Physiol Heart Circ Physiol. 2017 Oct 1;313(4):H687-H689. doi: 10.1152/ajpheart.00374.2017. Epub 2017 Jul 14. Am J Physiol Heart Circ Physiol. 2017. PMID: 28710071 Free PMC article. No abstract available.
Similar articles
-
Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and -9 in hypertensive pregnancy.Am J Physiol Heart Circ Physiol. 2020 Jan 1;318(1):H165-H180. doi: 10.1152/ajpheart.00602.2019. Epub 2019 Dec 13. Am J Physiol Heart Circ Physiol. 2020. PMID: 31834839 Free PMC article.
-
Placental growth factor reverses decreased vascular and uteroplacental MMP-2 and MMP-9 and increased MMP-1 and MMP-7 and collagen types I and IV in hypertensive pregnancy.Am J Physiol Heart Circ Physiol. 2018 Jul 1;315(1):H33-H47. doi: 10.1152/ajpheart.00045.2018. Epub 2018 Mar 23. Am J Physiol Heart Circ Physiol. 2018. PMID: 29569955 Free PMC article.
-
TNFα blockade reverses vascular and uteroplacental matrix metalloproteinases imbalance and collagen accumulation in hypertensive pregnant rats.Biochem Pharmacol. 2021 Nov;193:114790. doi: 10.1016/j.bcp.2021.114790. Epub 2021 Oct 1. Biochem Pharmacol. 2021. PMID: 34600915 Free PMC article.
-
Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia.Prog Mol Biol Transl Sci. 2017;148:87-165. doi: 10.1016/bs.pmbts.2017.04.001. Epub 2017 May 22. Prog Mol Biol Transl Sci. 2017. PMID: 28662830 Free PMC article. Review.
-
Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia.Biochem Pharmacol. 2015 Jun 15;95(4):211-26. doi: 10.1016/j.bcp.2015.04.012. Epub 2015 Apr 24. Biochem Pharmacol. 2015. PMID: 25916268 Free PMC article. Review.
Cited by
-
Mitochondria Targeted Antioxidant Significantly Alleviates Preeclampsia Caused by 11β-HSD2 Dysfunction via OPA1 and MtDNA Maintenance.Antioxidants (Basel). 2022 Jul 31;11(8):1505. doi: 10.3390/antiox11081505. Antioxidants (Basel). 2022. PMID: 36009224 Free PMC article.
-
Estrogen-induced FOS-like 1 regulates matrix metalloproteinase expression and the motility of human endometrial and decidual stromal cells.J Biol Chem. 2020 Feb 21;295(8):2248-2258. doi: 10.1074/jbc.RA119.010701. Epub 2020 Jan 14. J Biol Chem. 2020. PMID: 31937587 Free PMC article.
-
Increased matrix metalloproteinases in cerebrospinal fluids of patients with major depressive disorder and schizophrenia.Int J Neuropsychopharmacol. 2020 Jul 16;23(11):713-20. doi: 10.1093/ijnp/pyaa049. Online ahead of print. Int J Neuropsychopharmacol. 2020. PMID: 32671384 Free PMC article.
-
Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and -9 in hypertensive pregnancy.Am J Physiol Heart Circ Physiol. 2020 Jan 1;318(1):H165-H180. doi: 10.1152/ajpheart.00602.2019. Epub 2019 Dec 13. Am J Physiol Heart Circ Physiol. 2020. PMID: 31834839 Free PMC article.
-
Disturbed Cardiorespiratory Adaptation in Preeclampsia: Return to Normal Stress Regulation Shortly after Delivery?Int J Mol Sci. 2019 Jun 27;20(13):3149. doi: 10.3390/ijms20133149. Int J Mol Sci. 2019. PMID: 31252672 Free PMC article.
References
-
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270: 5872–5876, 1995. doi:10.1074/jbc.270.11.5872. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

