Therapeutic effects of CSF1R-blocking antibodies in multiple myeloma
- PMID: 28626216
- PMCID: PMC5927777
- DOI: 10.1038/leu.2017.193
Therapeutic effects of CSF1R-blocking antibodies in multiple myeloma
Abstract
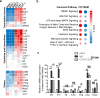
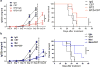
Our previous studies showed that macrophages (MФs), especially myeloma-associated MФs (MAMs), induce chemoresistance in human myeloma. Here we explored the potential of targeting MФs, by using colony-stimulating factor 1 receptor (CSF1R)-blocking mAbs, to treat myeloma. Our results showed that CSF1R blockade specifically inhibited the differentiation, proliferation and survival of murine M2 MФs and MAMs, and repolarized MAMs towards M1-like MФs in vitro. CSF1R blockade alone inhibited myeloma growth in vivo, by partially depleting MAMs, polarizing MAMs to the M1 phenotype, and inducing a tumor-specific cytotoxic CD4+ T-cell response. Similarly, genetically depleting MФs in myeloma-bearing MMDTR mice retarded myeloma growth in vivo. Furthermore, the combination of CSF1R blockade and chemotherapy such as bortezomib or melphalan displayed an additive therapeutic efficacy against established myeloma. Finally, a fully human CSF1R blocking mAb, similar to its murine counterpart, was able to inhibit the differentiation, proliferation and survival of human MФs. Thus, this study provides the first direct in vivo evidence that MΦs and MAMs are indeed important for myeloma development and progression. Our results also suggest that targeting MAMs by CSF1R blocking mAbs may be promising methods to (re)sensitize myeloma cells to chemotherapy and promote anti-myeloma immune responses in patients.
Conflict of interest statement
Figures
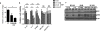
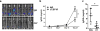
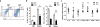

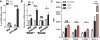
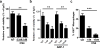
References
-
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007 Aug;7(8):585–598. - PubMed
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005 Dec;5(12):953–964. - PubMed
-
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010 Oct;11(10):889–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

