A phase 3 randomized placebo-controlled trial of darbepoetin alfa in patients with anemia and lower-risk myelodysplastic syndromes
- PMID: 28626220
- PMCID: PMC5596208
- DOI: 10.1038/leu.2017.192
A phase 3 randomized placebo-controlled trial of darbepoetin alfa in patients with anemia and lower-risk myelodysplastic syndromes
Abstract
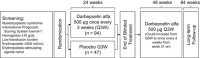
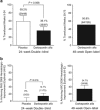
The use of darbepoetin alfa to treat anemia in patients with lower-risk myelodysplastic syndromes (MDS) was evaluated in a phase 3 trial. Eligible patients had low/intermediate-1 risk MDS, hemoglobin ⩽10 g/dl, low transfusion burden and serum erythropoietin (EPO) ⩽500 mU/ml. Patients were randomized 2:1 to receive 24 weeks of subcutaneous darbepoetin alfa 500 μg or placebo every 3 weeks (Q3W), followed by 48 weeks of open-label darbepoetin alfa. A total of 147 patients were randomized, with median hemoglobin of 9.3 (Q1:8.8, Q3:9.7) g/dl and median baseline serum EPO of 69 (Q1:36, Q3:158) mU/ml. Transfusion incidence from weeks 5-24 was significantly lower with darbepoetin alfa versus placebo (36.1% (35/97) versus 59.2% (29/49), P=0.008) and erythroid response rates increased significantly with darbepoetin alfa (14.7% (11/75 evaluable) versus 0% (0/35 evaluable), P=0.016). In the 48-week open-label period, dose frequency increased from Q3W to Q2W in 81% (102/126) of patients; this was associated with a higher hematologic improvement-erythroid response rate (34.7% (34/98)). Safety results were consistent with a previous darbepoetin alfa phase 2 MDS trial. In conclusion, 24 weeks of darbepoetin alfa Q3W significantly reduced transfusions and increased rates of erythroid response with no new safety signals in lower-risk MDS (registered as EudraCT#2009-016522-14 and NCT#01362140).
Conflict of interest statement
UP: honoraria from Amgen Inc., Celgene, Janssen and Novartis. AS: research grants from Amgen Inc. ENO: honoraria from Celgene, Amgen Inc., La Jolla and speakers’ bureau for Novartis, Celgene. JSG: honoraria from Amgen Inc. MD: honoraria from Amgen Inc. JM: research grants from Amgen Inc. BS: none. SB, BM and JF: employees and stockholders of Amgen Inc. EG: Novartis employee, former Amgen Inc. employee.
Figures

References
-
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088. - PubMed
-
- Platzbecker U, Hofbauer LC, Ehninger G, Holig K. The clinical, quality of life, and economic consequences of chronic anemia and transfusion support in patients with myelodysplastic syndromes. Leuk Res 2012; 36: 525–536. - PubMed
-
- National Comprehensive Cancer Network I. Myelodysplastic Syndromes. 2016 (cited 3 March 2016); Available from https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
-
- Onkologie DGfHuM. German S3 Leitlinie Guidelines. 2016 (cited 11 October 2016); Available from https://www.onkopedia.com/de/onkopedia/guidelines/myelodysplastische-syn....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

