Increasing N-acetylaspartate in the Brain during Postnatal Myelination Does Not Cause the CNS Pathologies of Canavan Disease
- PMID: 28626388
- PMCID: PMC5454052
- DOI: 10.3389/fnmol.2017.00161
Increasing N-acetylaspartate in the Brain during Postnatal Myelination Does Not Cause the CNS Pathologies of Canavan Disease
Abstract
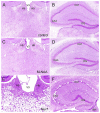
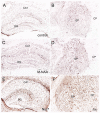
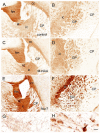
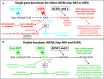
Canavan disease is caused by mutations in the gene encoding aspartoacylase (ASPA), a deacetylase that catabolizes N-acetylaspartate (NAA). The precise involvement of elevated NAA in the pathogenesis of Canavan disease is an ongoing debate. In the present study, we tested the effects of elevated NAA in the brain during postnatal development. Mice were administered high doses of the hydrophobic methyl ester of NAA (M-NAA) twice daily starting on day 7 after birth. This treatment increased NAA levels in the brain to those observed in the brains of Nur7 mice, an established model of Canavan disease. We evaluated various serological parameters, oxidative stress, inflammatory and neurodegeneration markers and the results showed that there were no pathological alterations in any measure with increased brain NAA levels. We examined oxidative stress markers, malondialdehyde content (indicator of lipid peroxidation), expression of NADPH oxidase and nuclear translocation of the stress-responsive transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF-2) in brain. We also examined additional pathological markers by immunohistochemistry and the expression of activated caspase-3 and interleukin-6 by Western blot. None of the markers were increased in the brains of M-NAA treated mice, and no vacuoles were observed in any brain region. These results show that ASPA expression prevents the pathologies associated with excessive NAA concentrations in the brain during postnatal myelination. We hypothesize that the pathogenesis of Canavan disease involves not only disrupted NAA metabolism, but also excessive NAA related signaling processes in oligodendrocytes that have not been fully determined and we discuss some of the potential mechanisms.
Keywords: Acss1; Acss2; NAA; NAAG; Nat8L; aspartoacylase; vacuoles; vacuolization.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

