The Effects of Blast Exposure on Protein Deimination in the Brain
- PMID: 28626499
- PMCID: PMC5463117
- DOI: 10.1155/2017/8398072
The Effects of Blast Exposure on Protein Deimination in the Brain
Abstract
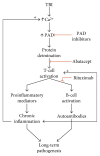
Oxidative stress and calcium excitotoxicity are hallmarks of traumatic brain injury (TBI). While these early disruptions may be corrected over a relatively short period of time, long-lasting consequences of TBI including impaired cognition and mood imbalances can persist for years, even in the absence of any evidence of overt injury based on neuroimaging. This investigation examined the possibility that disordered protein deimination occurs as a result of TBI and may thus contribute to the long-term pathologies of TBI. Protein deimination is a calcium-activated, posttranslational modification implicated in the autoimmune diseases rheumatoid arthritis and multiple sclerosis, where aberrant deimination creates antigenic epitopes that elicit an autoimmune attack. The present study utilized proteomic analyses to show that blast TBI alters the deimination status of proteins in the porcine cerebral cortex. The affected proteins represent a small subset of the entire brain proteome and include glial fibrillary acidic protein and vimentin, proteins reported to be involved in autoimmune-based pathologies. The data also indicate that blast injury is associated with an increase in immunoglobulins in the brain, possibly representing autoantibodies directed against novel protein epitopes. These findings indicate that aberrant protein deimination is a biomarker for blast TBI and may therefore underlie chronic neuropathologies of head injury.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

