Electrostatically driven resonance energy transfer in "cationic" biocompatible indium phosphide quantum dots
- PMID: 28626557
- PMCID: PMC5465571
- DOI: 10.1039/c7sc00592j
Electrostatically driven resonance energy transfer in "cationic" biocompatible indium phosphide quantum dots
Abstract
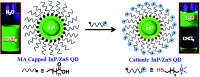
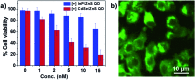
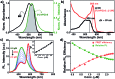
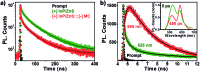
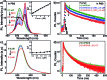
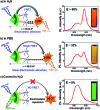
Indium Phosphide Quantum Dots (InP QDs) have emerged as an alternative to toxic metal ion based QDs in nanobiotechnology. The ability to generate cationic surface charge, without compromising stability and biocompatibility, is essential in realizing the full potential of InP QDs in biological applications. We have addressed this challenge by developing a place exchange protocol for the preparation of cationic InP/ZnS QDs. The quaternary ammonium group provides the much required permanent positive charge and stability to InP/ZnS QDs in biofluids. The two important properties of QDs, namely bioimaging and light induced resonance energy transfer, are successfully demonstrated in cationic InP/ZnS QDs. The low cytotoxicity and stable photoluminescence of cationic InP/ZnS QDs inside cells make them ideal candidates as optical probes for cellular imaging. An efficient resonance energy transfer (E ∼ 60%) is observed, under physiological conditions, between the cationic InP/ZnS QD donor and anionic dye acceptor. A large bimolecular quenching constant along with a linear Stern-Volmer plot confirms the formation of a strong ground state complex between the cationic InP/ZnS QDs and the anionic dye. Control experiments prove the role of electrostatic attraction in driving the light induced interactions, which can rightfully form the basis for future nano-bio studies between cationic InP/ZnS QDs and anionic biomolecules.
Figures
Similar articles
-
Blue-emitting InP quantum dots participate in an efficient resonance energy transfer process in water.Chem Sci. 2023 Apr 20;14(19):5167-5176. doi: 10.1039/d3sc00164d. eCollection 2023 May 17. Chem Sci. 2023. PMID: 37206393 Free PMC article.
-
Electrostatically Driven Resonance Energy Transfer in an All-Quantum Dot Based Donor-Acceptor System.J Phys Chem Lett. 2020 Jul 2;11(13):5354-5360. doi: 10.1021/acs.jpclett.0c01360. Epub 2020 Jun 22. J Phys Chem Lett. 2020. PMID: 32539403
-
Highly Bright Silica-Coated InP/ZnS Quantum Dot-Embedded Silica Nanoparticles as Biocompatible Nanoprobes.Int J Mol Sci. 2022 Sep 19;23(18):10977. doi: 10.3390/ijms231810977. Int J Mol Sci. 2022. PMID: 36142888 Free PMC article.
-
Comprehensive study of interaction between biocompatible PEG-InP/ZnS QDs and bovine serum albumin.Luminescence. 2018 May;33(3):495-504. doi: 10.1002/bio.3438. Epub 2017 Dec 28. Luminescence. 2018. PMID: 29282888
-
Luminescent quantum dots: Synthesis, optical properties, bioimaging and toxicity.Adv Drug Deliv Rev. 2023 Jun;197:114830. doi: 10.1016/j.addr.2023.114830. Epub 2023 Apr 20. Adv Drug Deliv Rev. 2023. PMID: 37086917 Review.
Cited by
-
Molecular insights and future frontiers in cell photosensitization for solar-driven CO2 conversion.iScience. 2021 Aug 5;24(9):102952. doi: 10.1016/j.isci.2021.102952. eCollection 2021 Sep 24. iScience. 2021. PMID: 34458701 Free PMC article. Review.
-
Solvation of quantum dots in 1-alkyl-1-methylpyrrolidinium ionic liquids: toward stably luminescent composites.Sci Technol Adv Mater. 2020 Mar 19;21(1):187-194. doi: 10.1080/14686996.2020.1735923. eCollection 2020. Sci Technol Adv Mater. 2020. PMID: 32284768 Free PMC article.
-
Blue-emitting InP quantum dots participate in an efficient resonance energy transfer process in water.Chem Sci. 2023 Apr 20;14(19):5167-5176. doi: 10.1039/d3sc00164d. eCollection 2023 May 17. Chem Sci. 2023. PMID: 37206393 Free PMC article.
-
Synergistic interactions of cadmium-free quantum dots embedded in a photosensitised polymer surface: efficient killing of multidrug-resistant strains at low ambient light levels.Nanoscale. 2020 May 21;12(19):10609-10622. doi: 10.1039/c9nr10421f. Epub 2020 May 6. Nanoscale. 2020. PMID: 32373810 Free PMC article.
-
Optoelectronic Neural Interfaces Based on Quantum Dots.ACS Appl Mater Interfaces. 2022 May 11;14(18):20468-20490. doi: 10.1021/acsami.1c25009. Epub 2022 Apr 28. ACS Appl Mater Interfaces. 2022. PMID: 35482955 Free PMC article. Review.
References
-
- Bajaj A., Rana S., Miranda O. R., Yawe J. C., Jerry D. J., Bunz U. H. F., Rotello V. M. Chem. Sci. 2010;1:134.
- Nel A. E., Mädler L., Velegol D., Xia T., Hoek E. M. V., Somasundaran P., Klaessig F., Castranova V., Thompson M. Nat. Mater. 2009;8:543. - PubMed
- Verma A., Stellacci F. Small. 2010;6:12. - PubMed
- Cho E. C., Au L., Zhang Q., Xia Y. Small. 2010;6:517. - PMC - PubMed
- Albanese A., Tang P. S., Chan W. C. W. Annu. Rev. Biomed. Eng. 2012;14:1. - PubMed
- Pillai P. P., Kowalczyk B., Kandere-Grzybowska K., Borkowska M., Grzybowski B. A. Angew. Chem., Int. Ed. 2016;55:8610. - PubMed
-
- Goodman C. M., McCusker C. D., Yilmaz T., Rotello V. M. Bioconjugate Chem. 2004;15:897. - PubMed
- Saha K., Bajaj A., Duncan B., Rotello V. M. Small. 2011;7:1903. - PMC - PubMed
- Zhu Z.-J., Yeh Y.-C., Tang R., Yan B., Tamayo J., Vachet R. W., Rotello V. M. Nat. Chem. 2011;3:963. - PMC - PubMed
- Ramos J., Forcada J., Hidalgo-Alvarez R. Chem. Rev. 2014;114:367. - PubMed
- Li L., Liu J., Yang X., Peng Z., Liu W., Xu J., Tang J., He X., Wang K. Chem. Commun. 2015;51:14357. - PubMed
-
- Dubertret B., Skourides P., Norris D. J., Noireaux V., Brivanlou A. H., Libchaber A. Science. 2002;298:1759. - PubMed
- Gao X., Cui Y., Levenson R. M., Chung L. W. K., Nie S. Nat. Biotechnol. 2004;22:969. - PubMed
- Medintz I. L., Uyeda H. T., Goldman E. R., Mattoussi H. Nat. Mater. 2005;4:435. - PubMed
- Choi H. S., Liu W., Misra P., Tanaka E., Zimmer J. P., Ipe B. I., Bawendi M. G., Frangioni J. V. Nat. Biotechnol. 2007;25:1165. - PMC - PubMed
- Liu W., Howarth M., Greytak A. B., Zheng Y., Nocera D. G., Ting A. Y., Bawendi M. G. J. Am. Chem. Soc. 2008;130:1274. - PMC - PubMed
- Biju V., Itoh T., Ishikawa M. Chem. Soc. Rev. 2010;39:3031. - PubMed
- Shibu E. S., Sugino S., Ono K., Saito H., Nishioka A., Yamamura S., Sawada M., Nosaka Y., Biju V. Angew. Chem., Int. Ed. 2013;52:10559. - PubMed
- Wegner K. D., Hildebrandt N. Chem. Soc. Rev. 2015;44:4792. - PubMed
-
- Clapp A. R., Medintz I. L., Mauro J. M., Fisher B. R., Bawendi M. G., Mattoussi H. J. Am. Chem. Soc. 2004;126:301. - PubMed
- Sapsford K. E., Berti L., Medintz I. L. Angew. Chem., Int. Ed. 2006;45:4562. - PubMed
- Funston A. M., Jasieniak J. J., Mulvaney P. Adv. Mater. 2008;20:4274.
- Medintz I. L., Mattoussi H. Phys. Chem. Chem. Phys. 2009;11:17. - PubMed
-
- Lu H., Schöps O., Woggon U., Niemeyer C. M. J. Am. Chem. Soc. 2008;130:4815. - PubMed
- Freeman R., Willner B., Willner I. J. Phys. Chem. Lett. 2011;2:2667.
- Biju V., Anas A., Akita H., Shibu E. S., Itoh T., Harashima H., Ishikawa M. ACS Nano. 2012;6:3776. - PubMed
- Jou A. F.-J., Lu C.-H., Ou Y.-C., Wang S.-S., Hsu S.-L., Willner I., Ho J.-A. A. Chem. Sci. 2015;6:659. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

