Riboflavin as a bioorthogonal photocatalyst for the activation of a PtIV prodrug
- PMID: 28626570
- PMCID: PMC5471451
- DOI: 10.1039/c7sc01109a
Riboflavin as a bioorthogonal photocatalyst for the activation of a PtIV prodrug
Abstract
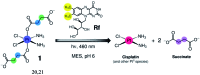
Encouraging developments demonstrate that few transition metal and organometallic catalysts can operate in a bioorthogonal fashion and promote non-natural chemistry in living systems by minimizing undesired side reactions with cellular components. These catalytic processes have potential for applications in medicinal chemistry and chemical biology. However, the stringent conditions of the cell environment severely limit the number of accessible metal catalysts and exogenous reactions. Herein, we report an unorthodox approach and a new type of bioorthogonal catalytic reaction, in which a metal complex is an unconventional substrate and an exogenous biological molecule acts as a catalyst. In this reaction, riboflavin photocatalytically converts a PtIV prodrug into cisplatin within the biological environment. Due to the catalytic activity of riboflavin, cisplatin-like apoptosis is induced in cancer cells under extremely low doses of light, potentially preventing systemic off-target reactions. Photocatalytic and bioorthogonal turnover of PtIV into PtII species is an attractive strategy to amplify the antineoplastic action of metal-based chemotherapeutics with spatio-temporal control.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

