Cancer drug delivery in the nano era: An overview and perspectives (Review)
- PMID: 28627697
- PMCID: PMC5562049
- DOI: 10.3892/or.2017.5718
Cancer drug delivery in the nano era: An overview and perspectives (Review)
Abstract
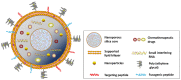
Nanomaterials are increasingly used as drug carriers for cancer therapy. Nanomaterials also appeal to researchers in the areas of cancer diagnosis and biomarker discovery. Several antitumor nanodrugs are currently being tested in preclinical and clinical trials and show promise in therapeutic and other settings. We review the development of nanomaterial drug carriers, including liposomes, polymer nanoparticles, dendritic polymers, and nanomicelles, for the diagnosis and treatment of various cancers. The prospects of nanomaterials as drug carriers for future clinical applications are also discussed.
Figures


References
-
- Shanthi M. Global Status Report on Noncommunicable Diseases 2014. Geneva: WHO Press, World Health Organization; 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

