The Sulfur-Linked Analogue of O-GlcNAc (S-GlcNAc) Is an Enzymatically Stable and Reasonable Structural Surrogate for O-GlcNAc at the Peptide and Protein Levels
- PMID: 28627871
- PMCID: PMC5598463
- DOI: 10.1021/acs.biochem.7b00268
The Sulfur-Linked Analogue of O-GlcNAc (S-GlcNAc) Is an Enzymatically Stable and Reasonable Structural Surrogate for O-GlcNAc at the Peptide and Protein Levels
Abstract
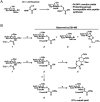
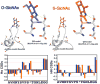
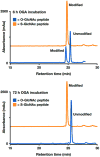
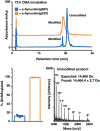
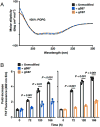
Synthetic proteins bearing site-specific posttranslational modifications have revolutionized our understanding of their biological functions in vitro and in vivo. One such modification, O-GlcNAcylation, is the dynamic addition of β-N-acetyl glucosamine to the side chains of serine and threonine residues of proteins, yet our understanding of the site-specific impact of O-GlcNAcylation remains difficult to evaluate in vivo because of the potential for enzymatic removal by endogenous O-GlcNAcase (OGA). Thioglycosides are generally perceived to be enzymatically stable structural mimics of O-GlcNAc; however, in vitro experiments with small-molecule GlcNAc thioglycosides have demonstrated that OGA can hydrolyze these linkages, indicating that S-linked β-N-acetyl glucosamine (S-GlcNAc) on peptides or proteins may not be completely stable. Here, we first develop a robust synthetic route to access an S-GlcNAcylated cysteine building block for peptide and protein synthesis. Using this modified amino acid, we establish that S-GlcNAc is an enzymatically stable surrogate for O-GlcNAcylation in its native protein setting. We also applied nuclear magnetic resonance spectroscopy and computational modeling to find that S-GlcNAc is an good structural mimic of O-GlcNAc. Finally, we demonstrate that site-specific S-GlcNAcylation results in biophysical characteristics that are the same as those of O-GlcNAc within the context of the protein α-synuclein. While this study is limited in focus to two model systems, these data suggest that S-GlcNAc broadly resembles O-GlcNAc and that it is indeed a stable analogue in the context of peptides and proteins.
Figures

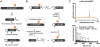
References
-
- Vocadlo DJ. O-GlcNAc processing enzymes: catalytic mechanisms, substrate specificity, and enzyme regulation. Curr Opin Chem Biol. 2012;16:488–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

