Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis
- PMID: 28628032
- PMCID: PMC5490765
- DOI: 10.1172/JCI92617
Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis
Abstract
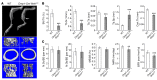
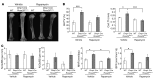
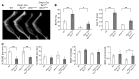
Mutations in WNT1 cause osteogenesis imperfecta (OI) and early-onset osteoporosis, identifying it as a key Wnt ligand in human bone homeostasis. However, how and where WNT1 acts in bone are unclear. To address this mechanism, we generated late-osteoblast-specific and osteocyte-specific WNT1 loss- and gain-of-function mouse models. Deletion of Wnt1 in osteocytes resulted in low bone mass with spontaneous fractures similar to that observed in OI patients. Conversely, Wnt1 overexpression from osteocytes stimulated bone formation by increasing osteoblast number and activity, which was due in part to activation of mTORC1 signaling. While antiresorptive therapy is the mainstay of OI treatment, it has limited efficacy in WNT1-related OI. In this study, anti-sclerostin antibody (Scl-Ab) treatment effectively improved bone mass and dramatically decreased fracture rate in swaying mice, a model of global Wnt1 loss. Collectively, our data suggest that WNT1-related OI and osteoporosis are caused in part by decreased mTORC1-dependent osteoblast function resulting from loss of WNT1 signaling in osteocytes. As such, this work identifies an anabolic function of osteocytes as a source of Wnt in bone development and homoeostasis, complementing their known function as targets of Wnt signaling in regulating osteoclastogenesis. Finally, this study suggests that Scl-Ab is an effective genotype-specific treatment option for WNT1-related OI and osteoporosis.
Conflict of interest statement
Figures



Comment in
-
Strengthening brittle bones.Sci Transl Med. 2017 Jun 28;9(396):eaan6730. doi: 10.1126/scitranslmed.aan6730. Sci Transl Med. 2017. PMID: 28659442
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

