Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity
- PMID: 28628040
- PMCID: PMC5490767
- DOI: 10.1172/JCI89934
Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity
Abstract
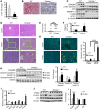
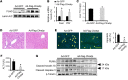
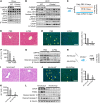
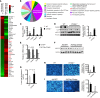
Epidemiologic and animal studies implicate overconsumption of fructose in the development of nonalcoholic fatty liver disease, but the molecular mechanisms underlying fructose-induced chronic liver diseases remain largely unknown. Here, we have presented evidence supporting the essential function of the lipogenic transcription factor carbohydrate response element-binding protein (ChREBP) in mediating adaptive responses to fructose and protecting against fructose-induced hepatotoxicity. In WT mice, a high-fructose diet (HFrD) activated hepatic lipogenesis in a ChREBP-dependent manner; however, in Chrebp-KO mice, a HFrD induced steatohepatitis. In Chrebp-KO mouse livers, a HFrD reduced levels of molecular chaperones and activated the C/EBP homologous protein-dependent (CHOP-dependent) unfolded protein response, whereas administration of a chemical chaperone or Chop shRNA rescued liver injury. Elevated expression levels of cholesterol biosynthesis genes in HFrD-fed Chrebp-KO livers were paralleled by an increased nuclear abundance of sterol regulatory element-binding protein 2 (SREBP2). Atorvastatin-mediated inhibition of hepatic cholesterol biosynthesis or depletion of hepatic Srebp2 reversed fructose-induced liver injury in Chrebp-KO mice. Mechanistically, we determined that ChREBP binds to nuclear SREBP2 to promote its ubiquitination and destabilization in cultured cells. Therefore, our findings demonstrate that ChREBP provides hepatoprotection against a HFrD by preventing overactivation of cholesterol biosynthesis and the subsequent CHOP-mediated, proapoptotic unfolded protein response. Our findings also identified a role for ChREBP in regulating SREBP2-dependent cholesterol metabolism.
Conflict of interest statement
Figures

Comment in
- ChREBP refines the hepatic response to fructose to protect the liver from injury doi: 10.1172/JCI95008
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

