Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber
- PMID: 28628101
- PMCID: PMC5533215
- DOI: 10.1038/nn.4591
Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber
Abstract
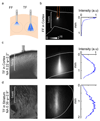
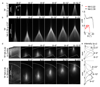
Optogenetics promises precise spatiotemporal control of neural processes using light. However, the spatial extent of illumination within the brain is difficult to control and cannot be adjusted using standard fiber optics. We demonstrate that optical fibers with tapered tips can be used to illuminate either spatially restricted or large brain volumes. Remotely adjusting the light input angle to the fiber varies the light-emitting portion of the taper over several millimeters without movement of the implant. We use this mode to activate dorsal versus ventral striatum of individual mice and reveal different effects of each manipulation on motor behavior. Conversely, injecting light over the full numerical aperture of the fiber results in light emission from the entire taper surface, achieving broader and more efficient optogenetic activation of neurons, compared to standard flat-faced fiber stimulation. Thus, tapered fibers permit focal or broad illumination that can be precisely and dynamically matched to experimental needs.
Conflict of interest statement
MDV, FP, BS, SD, and LS, authors on this study, are co-founders of Optogenix LLC, a company based in Italy that produces and markets the tapered fibers described here.
Figures





References
-
- Lee J, Ozden I, Song Y-K, Nurmikko AV. Nat Meth. advance online publication; 2015. Transparent intracortical microprobe array for simultaneous spatiotemporal optical stimulation and multichannel electrical recording. - PubMed
-
- Canales A, et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotech. 2015;33:277–284. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

