Therapeutic administration of a recombinant human monoclonal antibody reduces the severity of chikungunya virus disease in rhesus macaques
- PMID: 28628616
- PMCID: PMC5491320
- DOI: 10.1371/journal.pntd.0005637
Therapeutic administration of a recombinant human monoclonal antibody reduces the severity of chikungunya virus disease in rhesus macaques
Abstract
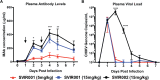
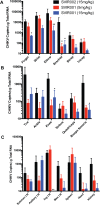
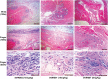
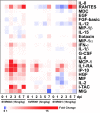
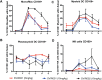
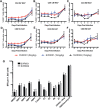
Chikungunya virus (CHIKV) is a mosquito-borne virus that causes a febrile syndrome in humans associated with acute and chronic debilitating joint and muscle pain. Currently no licensed vaccines or therapeutics are available to prevent or treat CHIKV infections. We recently isolated a panel of potently neutralizing human monoclonal antibodies (mAbs), one (4N12) of which exhibited prophylactic and post-exposure therapeutic activity against CHIKV in immunocompromised mice. Here, we describe the development of an engineered CHIKV mAb, designated SVIR001, that has similar antigen binding and neutralization profiles to its parent, 4N12. Because therapeutic administration of SVIR001 in immunocompetent mice significantly reduced viral load in joint tissues, we evaluated its efficacy in a rhesus macaque model of CHIKV infection. Rhesus macaques that were treated after infection with SVIR001 showed rapid elimination of viremia and less severe joint infiltration and disease compared to animals treated with SVIR002, an isotype control mAb. SVIR001 reduced viral burden at the site of infection and at distant sites and also diminished the numbers of activated innate immune cells and levels of pro-inflammatory cytokines and chemokines. SVIR001 therapy; however, did not substantively reduce the induction of CHIKV-specific B or T cell responses. Collectively, these results show promising therapeutic activity of a human anti-CHIKV mAb in rhesus macaques and provide proof-of-principle for its possible use in humans to treat active CHIKV infections.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: CC, SK, MM, PC, JR, ER, IF, and KC have ownership of stocks or shares in Sanofi. The rest of the authors have declared that no competing interests exist.
Figures

Similar articles
-
Antiviral Functions of Monoclonal Antibodies against Chikungunya Virus.Viruses. 2019 Mar 28;11(4):305. doi: 10.3390/v11040305. Viruses. 2019. PMID: 30925717 Free PMC article. Review.
-
Chikungunya viruses that escape monoclonal antibody therapy are clinically attenuated, stable, and not purified in mosquitoes.J Virol. 2014 Aug;88(15):8213-26. doi: 10.1128/JVI.01032-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829346 Free PMC article.
-
A potent neutralizing IgM mAb targeting the N218 epitope on E2 protein protects against Chikungunya virus pathogenesis.MAbs. 2015;7(6):1178-94. doi: 10.1080/19420862.2015.1083664. Epub 2015 Aug 25. MAbs. 2015. PMID: 26305993 Free PMC article.
-
A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection.Sci Immunol. 2019 May 17;4(35):eaaw6647. doi: 10.1126/sciimmunol.aaw6647. Sci Immunol. 2019. PMID: 31101672 Free PMC article.
-
Disease Resolution in Chikungunya-What Decides the Outcome?Front Immunol. 2020 Apr 28;11:695. doi: 10.3389/fimmu.2020.00695. eCollection 2020. Front Immunol. 2020. PMID: 32411133 Free PMC article. Review.
Cited by
-
Understanding the Biology and Immune Pathogenesis of Chikungunya Virus Infection for Diagnostic and Vaccine Development.Viruses. 2022 Dec 23;15(1):48. doi: 10.3390/v15010048. Viruses. 2022. PMID: 36680088 Free PMC article. Review.
-
Chikungunya Virus Vaccines: Platforms, Progress, and Challenges.Curr Top Microbiol Immunol. 2022;435:81-106. doi: 10.1007/82_2019_175. Curr Top Microbiol Immunol. 2022. PMID: 31338593 Review.
-
Antiviral Functions of Monoclonal Antibodies against Chikungunya Virus.Viruses. 2019 Mar 28;11(4):305. doi: 10.3390/v11040305. Viruses. 2019. PMID: 30925717 Free PMC article. Review.
-
Clinical Characteristics, Histopathology, and Tissue Immunolocalization of Chikungunya Virus Antigen in Fatal Cases.Clin Infect Dis. 2021 Jul 15;73(2):e345-e354. doi: 10.1093/cid/ciaa837. Clin Infect Dis. 2021. PMID: 32615591 Free PMC article.
-
Pan-protective anti-alphavirus human antibodies target a conserved E1 protein epitope.Cell. 2021 Aug 19;184(17):4414-4429.e19. doi: 10.1016/j.cell.2021.07.006. Cell. 2021. PMID: 34416146 Free PMC article.
References
-
- Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, Crublet E, et al. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature. 2010;468(7324):709–12. doi: 10.1038/nature09555 . - DOI - PubMed
-
- Schilte C, Staikowsky F, Staikovsky F, Couderc T, Madec Y, Carpentier F, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7(3):e2137 doi: 10.1371/journal.pntd.0002137 ; PubMed Central PMCID: PMCPMC3605278. - DOI - PMC - PubMed
-
- Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184(10):5914–27. doi: 10.4049/jimmunol.0900255 - DOI - PubMed
-
- Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, et al. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009;3(3):e389 doi: 10.1371/journal.pntd.0000389 ; PubMed Central PMCID: PMCPMC2647734. - DOI - PMC - PubMed
-
- Gerardin P, Guernier V, Perrau J, Fianu A, Le Roux K, Grivard P, et al. Estimating Chikungunya prevalence in La Reunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis. 2008;8:99 doi: 10.1186/1471-2334-8-99 ; PubMed Central PMCID: PMC2528011. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

