The human cytomegalovirus glycoprotein pUL11 acts via CD45 to induce T cell IL-10 secretion
- PMID: 28628650
- PMCID: PMC5491327
- DOI: 10.1371/journal.ppat.1006454
The human cytomegalovirus glycoprotein pUL11 acts via CD45 to induce T cell IL-10 secretion
Abstract
Human Cytomegalovirus (HCMV) is a widespread pathogen, infection with which can cause severe disease for immunocompromised individuals. The complex changes wrought on the host's immune system during both productive and latent HCMV infection are well known. Infected cells are masked and manipulated and uninfected immune cells are also affected; peripheral blood mononuclear cell (PBMC) proliferation is reduced and cytokine profiles altered. Levels increase of the anti-inflammatory cytokine IL-10, which may be important for the establishment of HCMV infections and is required for the development of high viral titres by murine cytomegalovirus. The mechanisms by which HCMV affects T cell IL-10 secretion are not understood. We show here that treatment of PBMC with purified pUL11 induces IL-10 producing T cells as a result of pUL11 binding to the CD45 phosphatase on T cells. IL-10 production induced by HCMV infection is also in part mediated by pUL11. Supernatants from pUL11 treated cells have anti-inflammatory effects on untreated PBMC. Considering the mechanism, CD45 can be a positive or negative regulator of TCR signalling, depending on its expression level, and we show that pUL11 also has concentration dependent activating or inhibitory effects on T cell proliferation and on the kinase function of the CD45 substrate Lck. pUL11 is therefore the first example of a viral protein that can target CD45 to induce T cells with anti-inflammatory properties. It is also the first HCMV protein shown to induce T cell IL-10 secretion. Understanding the mechanisms by which pUL11-induced changes in signal strength influence T cell development and function may provide the basis for the development of novel antiviral treatments and therapies against immune pathologies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
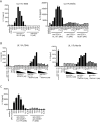


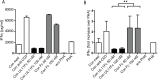
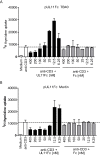
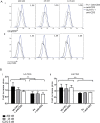


References
-
- Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in Medical Virology. 2010. pp. 202–213. doi: 10.1002/rmv.655 - DOI - PubMed
-
- Ludwig A, Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill. 2009;14: 26–32. - PubMed
-
- Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother. 2013;45: 260–71. doi: 10.3947/ic.2013.45.3.260 - DOI - PMC - PubMed
-
- Hebart H, Einsele H. Clinical aspects of CMV infection after stem cell transplantation. Human Immunology. 2004. pp. 432–436. doi: 10.1016/j.humimm.2004.02.022 - DOI - PubMed
-
- Randolph-Habecker J, Iwata M, Torok-Storb B. Cytomegalovirus mediated myelosuppression. J Clin Virol. 2002;25 Suppl 2: S51–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

