Hyperthermic intraperitoneal chemoperfusion as a component of multimodality therapy for ovarian and primary peritoneal cancer
- PMID: 28628712
- PMCID: PMC5533644
- DOI: 10.1002/jso.24666
Hyperthermic intraperitoneal chemoperfusion as a component of multimodality therapy for ovarian and primary peritoneal cancer
Abstract
Background and objectives: The role of hyperthermic intraperitoneal chemoperfusion (HIPEC) in the multimodality treatment of ovarian peritoneal metastases (OPM) and primary peritoneal cancer (PPC) remains controversial. We hypothesized that cytoreductive surgery (CRS) and HIPEC would provide meaningful survival benefit without excessive morbidity.
Methods: We reviewed clinicopathologic and perioperative data following 96 CRS-HIPEC procedures for primary or recurrent OPM and PPC. Kaplan-Meier survival curves and multivariate Cox-regression models identified prognostic factors affecting oncologic outcomes.
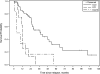
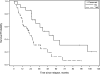
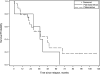
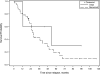
Results: CRS-HIPEC was mostly performed for recurrent disease (56.3%) and high-grade serous carcinoma (72.9%). Platinum-based systemic chemotherapy was administered to 89.5% of patients, with 75.5% having platinum-sensitive disease at CRS-HIPEC. Complete macroscopic resection was achieved in 70.8% of patients. Clavien-Dindo grade 3/4 morbidity occurred in 23.4% of patients; three patients died within 60-days postoperatively. Median overall survival from diagnosis of peritoneal metastases and CRS-HIPEC was 78 and 38 months, respectively. Completeness of cytoreduction, pathologic subtype, and 30-day morbidity were independent predictors of survival in multiple regression analysis.
Conclusions: Our study demonstrates promising survival data and supports the role of HIPEC in the multimodality treatment algorithm for primary or recurrent OPM and PPC. However definite indications and timing of HIPEC need to be clarified by prospective studies.
Keywords: HIPEC multimodality; cisplatin; cytoreductive surgery; ovarian cancer; primary peritoneal cancer.
© 2017 Wiley Periodicals, Inc.
Figures
References
-
- Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374(9698):1371–82. - PubMed
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351(24):2519–29. - PubMed
-
- Amate P, Huchon C, Dessapt AL, Bensaid C, Medioni J, Le Frere Belda MA, et al. Ovarian cancer: sites of recurrence. Int J Gynecol Cancer. 2013;23(9):1590–6. - PubMed
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. - PubMed
-
- Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

