Forced expiration measurements in mouse models of obstructive and restrictive lung diseases
- PMID: 28629359
- PMCID: PMC5477381
- DOI: 10.1186/s12931-017-0610-1
Forced expiration measurements in mouse models of obstructive and restrictive lung diseases
Abstract
Background: Pulmonary function measurements are important when studying respiratory disease models. Both resistance and compliance have been used to assess lung function in mice. Yet, it is not always clear how these parameters relate to forced expiration (FE)-related parameters, most commonly used in humans. We aimed to characterize FE measurements in four well-established mouse models of lung diseases.
Method: Detailed respiratory mechanics and FE measurements were assessed concurrently in Balb/c mice, using the forced oscillation and negative pressure-driven forced expiration techniques, respectively. Measurements were performed at baseline and following increasing methacholine challenges in control Balb/c mice as well as in four disease models: bleomycin-induced fibrosis, elastase-induced emphysema, LPS-induced acute lung injury and house dust mite-induced asthma.
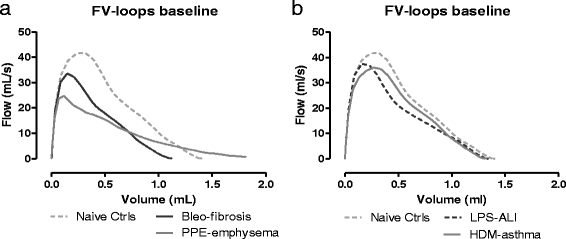
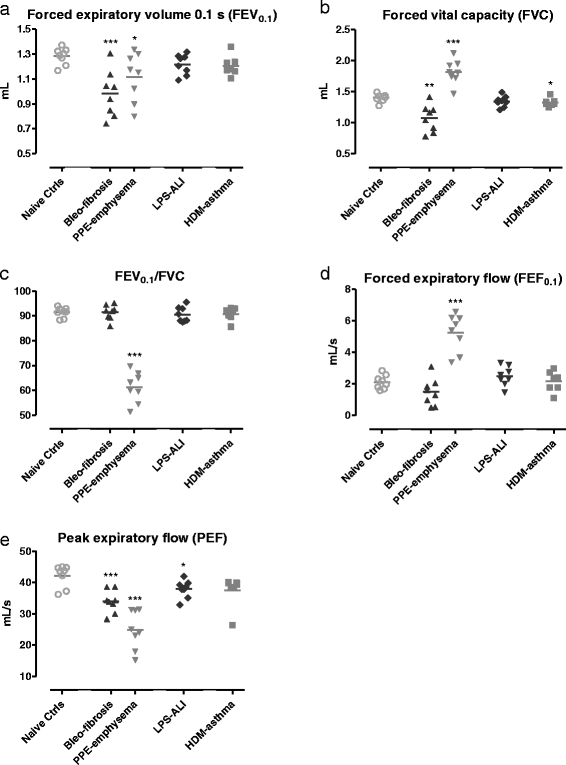
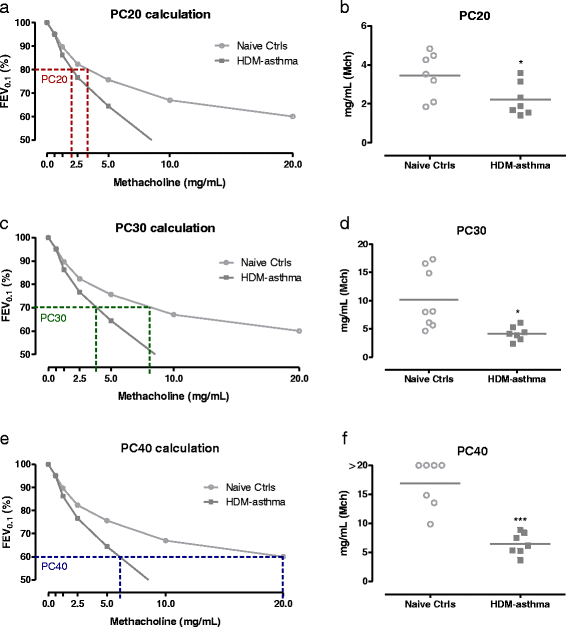
Results: Respiratory mechanics parameters (airway resistance, tissue damping and tissue elastance) confirmed disease-specific phenotypes either at baseline or following methacholine challenge. Similarly, lung function defects could be detected in each disease model by at least one FE-related parameter (FEV0.1, FEF0.1, FVC, FEV0.1/FVC ratio and PEF) at baseline or during the methacholine provocation assay.
Conclusions: FE-derived outcomes in four mouse disease models behaved similarly to changes found in human spirometry. Routine combined lung function assessments could increase the translational utility of mouse models.
Keywords: Acute lung injury; Airway resistance; Asthma; Emphysema; Fibrosis; Forced expiratory volume; Forced oscillations technique; Forced vital capacity; Mice; Negative pressure forced expiration; Tissue elastance.
Figures






References
-
- Hantos Z, Adamicza A, Govaerts E, Daroczy B. Mechanical impedances of lungs and chest wall in the cat. JApplPhysiol 1985. 1992;73:427–33. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

