Antiretroviral therapy initiation within seven days of enrolment: outcomes and time to undetectable viral load among children at an urban HIV clinic in Uganda
- PMID: 28629459
- PMCID: PMC5477116
- DOI: 10.1186/s12879-017-2550-2
Antiretroviral therapy initiation within seven days of enrolment: outcomes and time to undetectable viral load among children at an urban HIV clinic in Uganda
Abstract
Background: Viral suppression is a critical indicator of HIV treatment success. In the era of test-and-start, little is known about treatment outcomes and time to undetectable viral loads. This study compares treatment outcomes, median times to achieve undetectable viral loads and its predictors under different antiretroviral (ART) treatment initiation schedules (i.e. within seven days of enrolment or later).
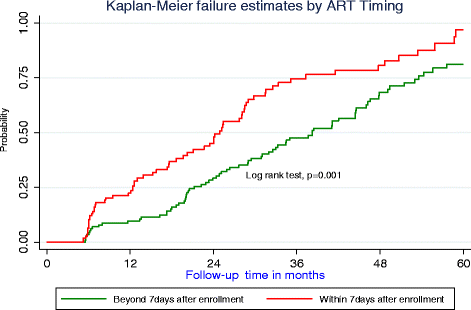
Methods: A retrospective cohort of 367 patients <18 years who enrolled in care between January 2010 and December 2015 with a baseline viral load of >5000 copies/ml were followed up for 60 months. Undetectable viral load measurements were based on both Roche (<20copies/ml) and Abbot (<75copies/ml). Clinical treatment outcomes were compared using chi-squared test. Survival experiences between the two cohorts were assessed through incidence rates and Kaplan Meier curves. A cox model with competing risks was used to assess predictors for time to undetectable viral load.
Results: Of the 367 patients, 180 (49.1%) initiated ART within seven days from enrolment, 192 (52.3%) attained undetectable viral load of which 133 (69.3%) were children below six years and 101 (52.6%) were females. Among those who initiated ART within seven days 15 (8.3%) died and 6 (3.3%) were lost to follow-up compared to 27 (14.4%) and 16 (8.6%) respectively in the later initiators. The median time to undetectable viral load was 24.9 months (95% CI: 19.7, 28.5) among early ART initiators and 38.5 months (95% CI: 31.1, 44.5) among those initiating beyond seven days. There was a significant difference in failure estimates between those initiating within seven and those that deferred (log rank, p = 0.001). Significant predictors for time to undetectable viral load were; starting ART within seven days (SHR = 2.02, 95% CI: 1.24, 3.28), baseline WHO stage I or II (SHR = 1.59, 95% CI: 1.06, 2.28), inconsistent adherence on three consecutive clinic visits (SHR = 0.44, 95% CI: 0.28, 0.67), and baseline weight (SRH = 1.04, 95% CI: 1.01, 1.07).
Conclusion: Prompt initiation of ART within the first week of enrolment is associated with better treatment outcomes. Early timing, baseline WHO clinical stage and adherence rates should be major considerations while managing HIV among children.
Keywords: Antiretroviral therapy; HIV; Outcomes; Timing; Undetectable viral load.
Figures
References
-
- UNAIDS, Global AIDS update 2016. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update... Accessed 15 July 2016.
-
- WHO, Accelerating progress on HIV, Tuberculosis, Malaria, Hepatitis and neglected tropical diseases. A new Agenda for 2016–2030. http://www.who.int/about/structure/organigram/htm/progress-hiv-tb-malari.... Accessed 21 June 2016.
-
- WHO, Antiretroviral Treatment as Prevention (TasP) of HIV and TB. 2012. http://www.who.int/hiv/pub/mtct/programmatic_update_tasp/en/. Accessed 21 June 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical