Emergency treatment with levetiracetam or phenytoin in status epilepticus in children-the EcLiPSE study: study protocol for a randomised controlled trial
- PMID: 28629473
- PMCID: PMC5477100
- DOI: 10.1186/s13063-017-2010-8
Emergency treatment with levetiracetam or phenytoin in status epilepticus in children-the EcLiPSE study: study protocol for a randomised controlled trial
Abstract
Background: Convulsive status epilepticus (CSE) is the most common life-threatening neurological emergency in childhood. These children are also at risk of significant morbidity, with acute and chronic impact on the family and the health and social care systems. The current recommended first-choice, second-line treatment in children aged 6 months and above is intravenous phenytoin (fosphenytoin in the USA), although there is a lack of evidence for its use and it is associated with significant side effects. Emerging evidence suggests that intravenous levetiracetam may be effective as a second-line agent for CSE, and fewer adverse effects have been described. This trial therefore aims to determine whether intravenous phenytoin or levetiracetam is more effective, and safer, in treating childhood CSE.
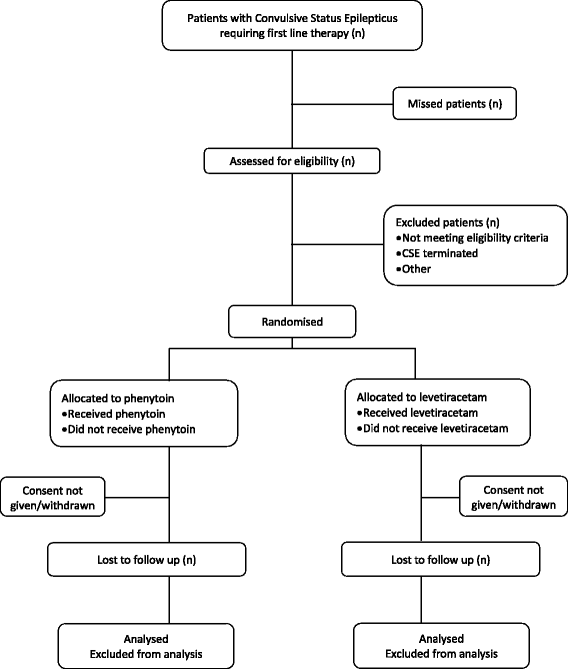
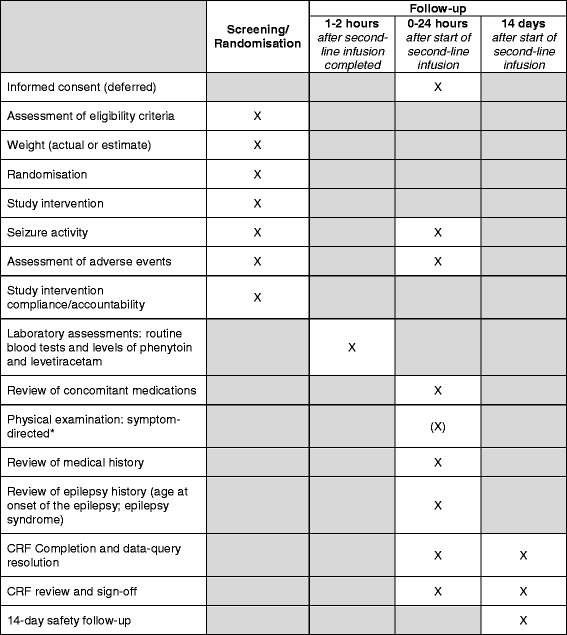
Methods/design: This is a phase IV, multi-centre, parallel group, randomised controlled, open-label trial. Following treatment for CSE with first-line treatment, children with ongoing seizures are randomised to receive either phenytoin (20 mg/kg, maximum 2 g) or levetiracetam (40 mg/kg, maximum 2.5 g) intravenously. The primary outcome measure is the cessation of all visible signs of CSE as determined by the treating clinician. Secondary outcome measures include the need for further anti-seizure medications or rapid sequence induction for ongoing CSE, admission to critical care areas, and serious adverse reactions. Patients are recruited without prior consent, with deferred consent sought at an appropriate time for the family. The primary analysis will be by intention-to-treat. The primary outcome is a time to event outcome and a sample size of 140 participants in each group will have 80% power to detect an increase in CSE cessation rates from 60% to 75%. Our total sample size of 308 randomised and treated participants will allow for 10% loss to follow-up.
Discussion: This clinical trial will determine whether phenytoin or levetiracetam is more effective as an intravenous second-line agent for CSE, and provide evidence for management recommendations. In addition, this trial will also provide data on which of these therapies is safer in this setting.
Trial registration: ISRCTN identifier, ISRCTN22567894 . Registered on 27 August 2015 EudraCT identifier, 2014-002188-13 . Registered on 21 May 2014 NIHR HTA Grant: 12/127/134.
Keywords: Levetiracetam; Paediatric; Phenytoin; Randomised; Seizure; Status epilepticus; Trial.
Figures

References
-
- PICANet annual report 2010. http://www.picanet.org.uk/Documentation/. Accessed 15 Dec 2016
-
- Advanced paediatric life support: a practical approach to emergencies (APLS) 6th edition. http://www.alsg.org/uk/Publications. Accessed 15 Dec 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

