Engineered myocardium model to study the roles of HIF-1α and HIF1A-AS1 in paracrine-only signaling under pathological level oxidative stress
- PMID: 28629892
- PMCID: PMC5563471
- DOI: 10.1016/j.actbio.2017.06.023
Engineered myocardium model to study the roles of HIF-1α and HIF1A-AS1 in paracrine-only signaling under pathological level oxidative stress
Abstract
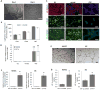
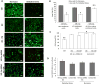
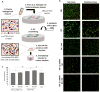
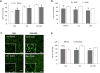
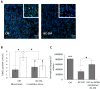
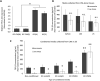
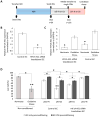
Studying heart tissue is critical for understanding and developing treatments for cardiovascular diseases. In this work, we fabricated precisely controlled and biomimetic engineered model tissues to study how cell-cell and cell-matrix interactions influence myocardial cell survival upon exposure to pathological level oxidative stress. Specifically, the interactions of endothelial cells (ECs) and cardiomyocytes (CMs), and the role of hypoxia inducible factor-1α (HIF-1α), with its novel alternative regulator, HIF-1α antisense RNA1 (HIF1A-AS1), in these interactions were investigated. We encapsulated CMs in photo-crosslinkable, biomimetic hydrogels with or without ECs, then exposed to oxidative stress followed by normoxia. With precisely controlled microenvironment provided by the model tissues, cell-cell interactions were restricted to be solely through the secreted factors. CM survival after oxidative stress was significantly improved, in the presence of ECs, when cells were in the model tissues that were functionalized with cell attachment motifs. Importantly, the cardioprotective effect of ECs was reduced when HIF-1α expression was knocked down suggesting that HIF-1α is involved in cardioprotection from oxidative damage, provided through secreted factors conferred by the ECs. Using model tissues, we showed that cell survival increased with increased cell-cell communication and enhanced cell-matrix interactions. In addition, whole genome transcriptome analysis showed, for the first time to our knowledge, a possible role for HIF1A-AS1 in oxidative regulation of HIF-1α. We showed that although HIF1A-AS1 knockdown helps CM survival, its effect is overridden by CM-EC bidirectional interactions as we showed that the conditioned media taken from the CM-EC co-cultures improved CM survival, regardless of HIF1A-AS1 expression.
Statement of significance: Cardiovascular diseases, most of which are associated with oxidative stress, is the most common cause of death worldwide. Thus, understanding the molecular events as well as the role of intercellular communication under oxidative stress is upmost importance in its prevention. In this study we used 3D engineered tissue models to investigate the role of HIF-1α and its regulation in EC-mediated cardioprotection. We showed that EC-mediated protection is only possible when there is a bidirectional crosstalk between ECs and CMs even without physical cell-cell contact. In addition, this protective effect is at least partially related to cell-ECM interactions and HIF-1α, which is regulated by HIF1A-AS1 under oxidative stress.
Keywords: Cardiomyocyte; Endothelial cell; Hypoxia inducible factor; Oxidative stress; Tissue engineering.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Transcriptome profiling of 3D co-cultured cardiomyocytes and endothelial cells under oxidative stress using a photocrosslinkable hydrogel system.Acta Biomater. 2017 Aug;58:337-348. doi: 10.1016/j.actbio.2017.06.031. Epub 2017 Jun 23. Acta Biomater. 2017. PMID: 28648749 Free PMC article.
-
Inhibition of HIF1A-AS1 promoted starvation-induced hepatocellular carcinoma cell apoptosis by reducing HIF-1α/mTOR-mediated autophagy.World J Surg Oncol. 2020 May 30;18(1):113. doi: 10.1186/s12957-020-01884-x. World J Surg Oncol. 2020. PMID: 32473641 Free PMC article.
-
Endothelial Hypoxia-Inducible Factor-1α Promotes Atherosclerosis and Monocyte Recruitment by Upregulating MicroRNA-19a.Hypertension. 2015 Dec;66(6):1220-6. doi: 10.1161/HYPERTENSIONAHA.115.05886. Epub 2015 Oct 19. Hypertension. 2015. PMID: 26483345
-
Long Noncoding RNAs in Pathological Cardiac Remodeling: A Review of the Update Literature.Biomed Res Int. 2019 Jul 1;2019:7159592. doi: 10.1155/2019/7159592. eCollection 2019. Biomed Res Int. 2019. PMID: 31355277 Free PMC article. Review.
-
Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1α in cancer.Cancer Sci. 2019 May;110(5):1510-1517. doi: 10.1111/cas.13990. Epub 2019 Mar 23. Cancer Sci. 2019. PMID: 30844107 Free PMC article. Review.
Cited by
-
In vitro aged, hiPSC-origin engineered heart tissue models with age-dependent functional deterioration to study myocardial infarction.Acta Biomater. 2019 Aug;94:372-391. doi: 10.1016/j.actbio.2019.05.064. Epub 2019 May 27. Acta Biomater. 2019. PMID: 31146032 Free PMC article.
-
Adipose stem cell secretome markedly improves rodent heart and human induced pluripotent stem cell-derived cardiomyocyte recovery from cardioplegic transport solution exposure.Stem Cells. 2021 Feb;39(2):170-182. doi: 10.1002/stem.3296. Epub 2020 Dec 23. Stem Cells. 2021. PMID: 33159685 Free PMC article.
-
Advances in the design, generation, and application of tissue-engineered myocardial equivalents.Front Bioeng Biotechnol. 2023 Sep 22;11:1247572. doi: 10.3389/fbioe.2023.1247572. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37811368 Free PMC article. Review.
-
Transcriptome profiling of 3D co-cultured cardiomyocytes and endothelial cells under oxidative stress using a photocrosslinkable hydrogel system.Acta Biomater. 2017 Aug;58:337-348. doi: 10.1016/j.actbio.2017.06.031. Epub 2017 Jun 23. Acta Biomater. 2017. PMID: 28648749 Free PMC article.
-
Human Heart Anoxia and Reperfusion Tissue (HEART) Model for the Rapid Study of Exosome Bound miRNA Expression As Biomarkers for Myocardial Infarction.Small. 2022 Jul;18(28):e2201330. doi: 10.1002/smll.202201330. Epub 2022 Jun 7. Small. 2022. PMID: 35670145 Free PMC article.
References
-
- Carde DL, Granger DN. Pathophysiology of reperfusion injury. J Pathol. 2000;190:255–266. - PubMed
-
- Piper HM, Garcia-Dorado D, Ovize MA. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. - PubMed
-
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

