Pseudomonas Quinolone Signal Induces Oxidative Stress and Inhibits Heme Oxygenase-1 Expression in Lung Epithelial Cells
- PMID: 28630072
- PMCID: PMC5563587
- DOI: 10.1128/IAI.00176-17
Pseudomonas Quinolone Signal Induces Oxidative Stress and Inhibits Heme Oxygenase-1 Expression in Lung Epithelial Cells
Abstract
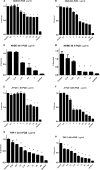
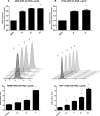
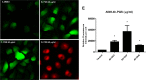
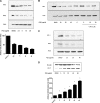
Pseudomonasaeruginosa causes lung infections in patients with cystic fibrosis (CF). The Pseudomonas quinolone signal (PQS) compound is a secreted P. aeruginosa virulence factor that contributes to the pathogenicity of P. aeruginosa We were able to detect PQS in sputum samples from CF patients infected with P. aeruginosa but not in samples from uninfected patients. We then tested the hypothesis that PQS induces oxidative stress in host cells by determining the ability of PQS to induce the production of reactive oxygen species (ROS) in lung epithelial cells (A549 and primary normal human bronchial epithelial [NHBE]) cells and macrophages (J774A.1 and THP-1). ROS production induced by PQS was detected with fluorescent probes (dichlorodihydrofluorescein diacetate, dihydroethidium, and MitoSOX Red) in conjunction with confocal microscopy and flow cytometry. PQS induced ROS production in lung epithelial (A549 and NHBE) cells and macrophages (J774A.1 and THP-1 cells). NHBE cells were sensitive to PQS concentrations as low as 500 ng/ml. PQS significantly induced early apoptosis (P < 0.05, n = 6) in lung epithelial cells, as measured by annexin/propidium iodide detection by flow cytometry. However, no change in apoptosis upon PQS treatment was seen in J774A.1 cells. Heme oxygenase-1 (HO-1) protein is an antioxidant enzyme usually induced by oxidative stress. Interestingly, incubation with PQS significantly reduced HO-1 and NrF2 expression in A549 and NHBE cells but increased HO-1 expression in J774A.1 cells (P < 0.05, n = 3), as determined by immunoblotting and densitometry. These PQS effects on host cells could play an important role in the pathogenicity of P. aeruginosa infections.
Keywords: PQS; Pseudomonas quinolone signal; cystic fibrosis; heme oxygenase-1; oxidative stress.
Copyright © 2017 American Society for Microbiology.
Figures


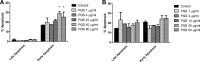
References
-
- Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O'Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL. 2011. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol 186:195–202. doi: 10.4049/jimmunol.1002104. - DOI - PMC - PubMed
-
- Pollack M. 2000. Pseudomonas aeruginosa, p 2310–2335. In Mandell GL, Bennett JE, Dolin R (ed), Principles and practice of infectious diseases, vol 5 Churchill Livingstone, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

