Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets
- PMID: 28630089
- PMCID: PMC5502437
- DOI: 10.1084/jem.20170494
Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets
Abstract
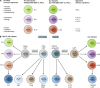
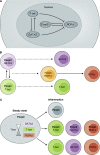
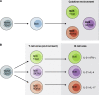
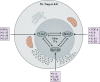
CD4 T cells, including T regulatory cells (Treg cells) and effector T helper cells (Th cells), and recently identified innate lymphoid cells (ILCs) play important roles in host defense and inflammation. Both CD4 T cells and ILCs can be classified into distinct lineages based on their functions and the expression of lineage-specific genes, including those encoding effector cytokines, cell surface markers, and key transcription factors. It was first recognized that each lineage expresses a specific master transcription factor and the expression of these factors is mutually exclusive because of cross-regulation among these factors. However, recent studies indicate that the master regulators are often coexpressed. Furthermore, the expression of master regulators can be dynamic and quantitative. In this review, we will first discuss similarities and differences between the development and functions of CD4 T cell and ILC subsets and then summarize recent literature on quantitative, dynamic, and cell type-specific balance between the master transcription factors in determining heterogeneity and plasticity of these subsets.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

