Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults
- PMID: 28630122
- PMCID: PMC5561903
- DOI: 10.1182/blood-2017-05-781450
Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults
Abstract
Eculizumab, a humanized anti-complement C5 monoclonal antibody (mAb) for treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome, blocks the terminal complement pathway required for serum bactericidal activity (SBA). Because treated patients are at >1000-fold increased risk of meningococcal disease, vaccination is recommended; whether vaccination can protect by opsonophagocytic activity in the absence of SBA is not known. Meningococci were added to anticoagulated blood from 12 healthy adults vaccinated with meningococcal serogroup B and serogroup A, C, W, Y vaccines. Bacterial survival was measured after 3-hour incubation in the presence of eculizumab or control complement factor D inhibitor ACH-4471, which blocks the complement alternative pathway (AP) and is in phase 2 development for treatment of PNH. In the absence of inhibitors, colony formation units (CFUs) per milliliter in blood from all 12 immunized subjects decreased from ∼4000 at time 0 to sterile cultures at 3 hours. In the presence of eculizumab, there was a >22-fold increase in geometric mean CFUs per milliliter (90 596 and 114 683 CFU/mL for serogroup B and C strains, respectively; P < .0001 compared with time 0). In the presence of ACH-4471, there was a >12-fold decrease (23 and 331 CFU/mL, respectively; P < .0001). The lack of meningococci killing by blood containing eculizumab resulted from inhibition of release of C5a, a C5 split product needed for upregulation of phagocytosis. The results provide an explanation for the large number of cases of meningococcal disease in immunized patients being treated with eculizumab and suggest that vaccination may provide better protection against meningococcal disease in patients treated with an AP-specific inhibitor.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.M.G. is an inventor on patent applications or on issued patents in the area of meningococcal vaccines. Rights to these inventions have been assigned to UCSF Benioff Children’s Hospital Oakland. M.K. declares no competing financial interests.
Figures


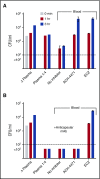
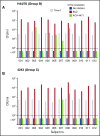
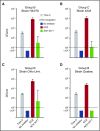
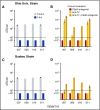
Comment in
-
Accept the complement (blockade).Blood. 2017 Aug 17;130(7):842-843. doi: 10.1182/blood-2017-07-793364. Blood. 2017. PMID: 28818977 No abstract available.
References
-
- Dmytrijuk A, Robie-Suh K, Cohen MH, Rieves D, Weiss K, Pazdur R. FDA report: eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist. 2008;13(9):993-1000. - PubMed
-
- Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8(11):643-657. - PubMed
-
- Sprong T, Brandtzaeg P, Fung M, et al. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood. 2003;102(10):3702-3710. - PubMed
-
- Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350(6):552-559. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

