Absence of the Polar Organizing Protein PopZ Results in Reduced and Asymmetric Cell Division in Agrobacterium tumefaciens
- PMID: 28630123
- PMCID: PMC5553032
- DOI: 10.1128/JB.00101-17
Absence of the Polar Organizing Protein PopZ Results in Reduced and Asymmetric Cell Division in Agrobacterium tumefaciens
Abstract
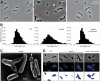
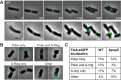
Agrobacterium tumefaciens is a rod-shaped bacterium that grows by polar insertion of new peptidoglycan during cell elongation. As the cell cycle progresses, peptidoglycan synthesis at the pole ceases prior to insertion of new peptidoglycan at midcell to enable cell division. The A. tumefaciens homolog of the Caulobacter crescentus polar organelle development protein PopZ has been identified as a growth pole marker and a candidate polar growth-promoting factor. Here, we characterize the function of PopZ in cell growth and division of A. tumefaciens Consistent with previous observations, we observe that PopZ localizes specifically to the growth pole in wild-type cells. Despite the striking localization pattern of PopZ, we find the absence of the protein does not impair polar elongation or cause major changes in the peptidoglycan composition. Instead, we observe an atypical cell length distribution, including minicells, elongated cells, and cells with ectopic poles. Most minicells lack DNA, suggesting a defect in chromosome segregation. Furthermore, the canonical cell division proteins FtsZ and FtsA are misplaced, leading to asymmetric sites of cell constriction. Together, these data suggest that PopZ plays an important role in the regulation of chromosome segregation and cell division.IMPORTANCEA. tumefaciens is a bacterial plant pathogen and a natural genetic engineer. However, very little is known about the spatial and temporal regulation of cell wall biogenesis that leads to polar growth in this bacterium. Understanding the molecular basis of A. tumefaciens growth may allow for the development of innovations to prevent disease or to promote growth during biotechnology applications. Finally, since many closely related plant and animal pathogens exhibit polar growth, discoveries in A. tumefaciens may be broadly applicable for devising antimicrobial strategies.
Keywords: Agrobacterium; PopZ; cell division; cell polarity; chromosome segregation; growth polarity.
Copyright © 2017 American Society for Microbiology.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

