Polar Organizing Protein PopZ Is Required for Chromosome Segregation in Agrobacterium tumefaciens
- PMID: 28630129
- PMCID: PMC5553026
- DOI: 10.1128/JB.00111-17
Polar Organizing Protein PopZ Is Required for Chromosome Segregation in Agrobacterium tumefaciens
Abstract
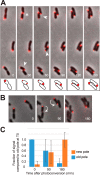
Despite being perceived as relatively simple organisms, many bacteria exhibit an impressive degree of subcellular organization. In Caulobacter crescentus, the evolutionarily conserved polar organizing protein PopZ facilitates cytoplasmic organization by recruiting chromosome centromeres and regulatory proteins to the cell poles. Here, we characterize the localization and function of PopZ in Agrobacterium tumefaciens, a genetically related species with distinct anatomy. In this species, we find that PopZ molecules are relocated from the old pole to the new pole in the minutes following cell division. PopZ is not required for the localization of the histidine kinases DivJ and PdhS1, which become localized to the old pole after PopZ relocation is complete. The histidine kinase PdhS2 is temporally and spatially related to PopZ in that it localizes to transitional poles just before they begin to shed PopZ and disappears from the old pole after PopZ relocalization. At the new pole, PopZ is required for tethering the centromere of at least one of multiple replicons (chromosome I), and the loss of popZ results in a severe chromosome segregation defect, aberrant cell division, and cell mortality. After cell division, the daughter that inherits polar PopZ is shorter in length and delayed in chromosome I segregation compared to its sibling. In this cell type, PopZ completes polar relocation well before the onset of chromosome segregation. While A. tumefaciens PopZ resembles its C. crescentus homolog in chromosome tethering activity, other aspects of its localization and function indicate distinct properties related to differences in cell organization.IMPORTANCE Members of the Alphaproteobacteria exhibit a wide range of phenotypic diversity despite sharing many conserved genes. In recent years, the extent to which this diversity is reflected at the level of subcellular organization has become increasingly apparent. However, which factors control such organization and how they have changed to suit different body plans are poorly understood. This study focuses on PopZ, which is essential for many aspects of polar organization in Caulobacter crescentus, but its role in other species is unclear. We explore the similarities and differences in PopZ functions between Agrobacterium tumefaciens and Caulobacter crescentus and conclude that PopZ lies at a point of diversification in the mechanisms that control cytoplasmic organization and cell cycle regulation in Alphaproteobacteria.
Keywords: Agrobacterium; PopZ; cell division; cell polarity; chromosome segregation.
Copyright © 2017 Ehrle et al.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

