Membrane-spanning α-helical barrels as tractable protein-design targets
- PMID: 28630153
- PMCID: PMC5483516
- DOI: 10.1098/rstb.2016.0213
Membrane-spanning α-helical barrels as tractable protein-design targets
Abstract
The rational (de novo) design of membrane-spanning proteins lags behind that for water-soluble globular proteins. This is due to gaps in our knowledge of membrane-protein structure, and experimental difficulties in studying such proteins compared to water-soluble counterparts. One limiting factor is the small number of experimentally determined three-dimensional structures for transmembrane proteins. By contrast, many tens of thousands of globular protein structures provide a rich source of 'scaffolds' for protein design, and the means to garner sequence-to-structure relationships to guide the design process. The α-helical coiled coil is a protein-structure element found in both globular and membrane proteins, where it cements a variety of helix-helix interactions and helical bundles. Our deep understanding of coiled coils has enabled a large number of successful de novo designs. For one class, the α-helical barrels-that is, symmetric bundles of five or more helices with central accessible channels-there are both water-soluble and membrane-spanning examples. Recent computational designs of water-soluble α-helical barrels with five to seven helices have advanced the design field considerably. Here we identify and classify analogous and more complicated membrane-spanning α-helical barrels from the Protein Data Bank. These provide tantalizing but tractable targets for protein engineering and de novo protein design.This article is part of the themed issue 'Membrane pores: from structure and assembly, to medicine and technology'.
Keywords: coiled coil; de novo protein design; transmembrane proteins; α-helical barrel.
© 2017 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures
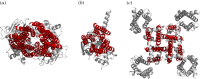
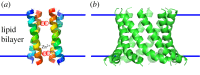
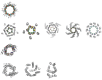
References
-
- Dingledine R, Borges K, Bowie D, Traynelis SF. 1999. The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61. - PubMed
-
- Luckey M. 2008. Membrane structural biology: with biochemical and biophysical foundations. Cambridge, UK: Cambridge University Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

