The mystery behind membrane insertion: a review of the complement membrane attack complex
- PMID: 28630159
- PMCID: PMC5483522
- DOI: 10.1098/rstb.2016.0221
The mystery behind membrane insertion: a review of the complement membrane attack complex
Abstract
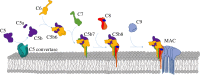
The membrane attack complex (MAC) is an important innate immune effector of the complement terminal pathway that forms cytotoxic pores on the surface of microbes. Despite many years of research, MAC structure and mechanism of action have remained elusive, relying heavily on modelling and inference from biochemical experiments. Recent advances in structural biology, specifically cryo-electron microscopy, have provided new insights into the molecular mechanism of MAC assembly. Its unique 'split-washer' shape, coupled with an irregular giant β-barrel architecture, enable an atypical mechanism of hole punching and represent a novel system for which to study pore formation. This review will introduce the complement terminal pathway that leads to formation of the MAC. Moreover, it will discuss how structures of the pore and component proteins underpin a mechanism for MAC function, modulation and inhibition.This article is part of the themed issue 'Membrane pores: from structure and assembly, to medicine and technology'.
Keywords: MACPF; cholesterol-dependent cytolysin; complement pathway; membrane attack complex; pore-forming protein; pore-forming toxins.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
-
- Harriman GR, Esser AF, Podack ER, Wunderlich AC, Braude AI, Lint TF, Curd JG. 1981. The role of C9 in complement-mediated killing of Neisseria. J. Immunol. 127, 2386–2390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
