De Novo Acquisition of Resistance to SCY-078 in Candida glabrata Involves FKS Mutations That both Overlap and Are Distinct from Those Conferring Echinocandin Resistance
- PMID: 28630180
- PMCID: PMC5571364
- DOI: 10.1128/AAC.00833-17
De Novo Acquisition of Resistance to SCY-078 in Candida glabrata Involves FKS Mutations That both Overlap and Are Distinct from Those Conferring Echinocandin Resistance
Abstract
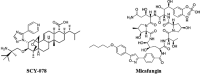
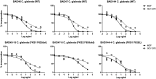
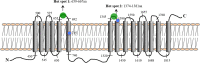
SCY-078 is an orally active antifungal whose target is the β-(1,3)-d-glucan synthase (GS). We evaluated the spontaneous emergence of SCY-078-resistant Candida glabrata isolates following drug exposure in vitro Resistant isolates were analyzed using broth microdilution methodology and FKS sequencing. The kinetic inhibition parameter IC50 (50% inhibitory concentration) was also determined from GS complexes. The spectrum of resistance mutations found suggested a partially overlapping but independent binding site for SCY-078 relative to echinocandins on GS.
Keywords: Candida glabrata; SCY-078; resistance.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Differential Activity of the Oral Glucan Synthase Inhibitor SCY-078 against Wild-Type and Echinocandin-Resistant Strains of Candida Species.Antimicrob Agents Chemother. 2017 Jul 25;61(8):e00161-17. doi: 10.1128/AAC.00161-17. Print 2017 Aug. Antimicrob Agents Chemother. 2017. PMID: 28533234 Free PMC article.
-
In Vitro Activity of Ibrexafungerp, a Novel Glucan Synthase Inhibitor against Candida glabrata Isolates with FKS Mutations.Antimicrob Agents Chemother. 2019 Oct 22;63(11):e01692-19. doi: 10.1128/AAC.01692-19. Print 2019 Nov. Antimicrob Agents Chemother. 2019. PMID: 31481447 Free PMC article.
-
Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations.Clin Infect Dis. 2013 Jun;56(12):1724-32. doi: 10.1093/cid/cit136. Epub 2013 Mar 13. Clin Infect Dis. 2013. PMID: 23487382 Free PMC article.
-
Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment.Am J Med. 2012 Jan;125(1 Suppl):S3-13. doi: 10.1016/j.amjmed.2011.11.001. Am J Med. 2012. PMID: 22196207 Review.
-
Clinical perspectives on echinocandin resistance among Candida species.Curr Opin Infect Dis. 2015 Dec;28(6):514-22. doi: 10.1097/QCO.0000000000000215. Curr Opin Infect Dis. 2015. PMID: 26524326 Free PMC article. Review.
Cited by
-
Enfumafungin synthase represents a novel lineage of fungal triterpene cyclases.Environ Microbiol. 2018 Sep;20(9):3325-3342. doi: 10.1111/1462-2920.14333. Epub 2018 Sep 13. Environ Microbiol. 2018. PMID: 30051576 Free PMC article.
-
Antifungal Resistance and the Role of New Therapeutic Agents.Curr Infect Dis Rep. 2022;24(9):105-116. doi: 10.1007/s11908-022-00782-5. Epub 2022 Jul 5. Curr Infect Dis Rep. 2022. PMID: 35812838 Free PMC article. Review.
-
Ibrexafungerp, a Novel Oral Triterpenoid Antifungal in Development: Overview of Antifungal Activity Against Candida glabrata.Front Cell Infect Microbiol. 2021 Mar 11;11:642358. doi: 10.3389/fcimb.2021.642358. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791244 Free PMC article. Review.
-
Ibrexafungerp: A narrative overview.Curr Res Microb Sci. 2024 May 27;6:100245. doi: 10.1016/j.crmicr.2024.100245. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 38873590 Free PMC article. Review.
-
In vitro activity of ibrexafungerp against clinically relevant echinocandin-resistant Candida strains.Antimicrob Agents Chemother. 2024 Feb 7;68(2):e0132423. doi: 10.1128/aac.01324-23. Epub 2024 Jan 11. Antimicrob Agents Chemother. 2024. PMID: 38206004 Free PMC article.
References
-
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr., Calandra TF, Edwards JE Jr., Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America . 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi:10.1086/596757. - DOI - PMC - PubMed
-
- Onishi J, Meinz M, Thompson J, Curotto J, Dreikorn S, Rosenbach M, Douglas C, Abruzzo G, Flattery A, Kong L, Cabello A, Vicente F, Pelaez F, Diez MT, Martin I, Bills G, Giacobbe R, Dombrowski A, Schwartz R, Morris S, Harris G, Tsipouras A, Wilson K, Kurtz MB. 2000. Discovery of novel antifungal (1,3)-beta-d-glucan synthase inhibitors. Antimicrob Agents Chemother 44:368–377. doi:10.1128/AAC.44.2.368-377.2000. - DOI - PMC - PubMed
-
- Jimenez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. 2014. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother 58:1248–1251. doi:10.1128/AAC.02145-13. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

