Triacylglycerol mimetics regulate membrane interactions of glycogen branching enzyme: implications for therapy
- PMID: 28630259
- PMCID: PMC5538282
- DOI: 10.1194/jlr.M075531
Triacylglycerol mimetics regulate membrane interactions of glycogen branching enzyme: implications for therapy
Abstract
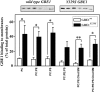
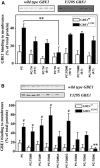
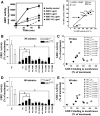
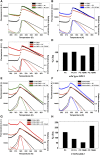
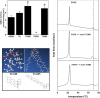
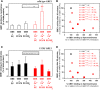
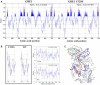
Adult polyglucosan body disease (APBD) is a neurological disorder characterized by adult-onset neurogenic bladder, spasticity, weakness, and sensory loss. The disease is caused by aberrant glycogen branching enzyme (GBE) (GBE1Y329S) yielding less branched, globular, and soluble glycogen, which tends to aggregate. We explore here whether, despite being a soluble enzyme, GBE1 activity is regulated by protein-membrane interactions. Because soluble proteins can contact a wide variety of cell membranes, we investigated the interactions of purified WT and GBE1Y329S proteins with different types of model membranes (liposomes). Interestingly, both triheptanoin and some triacylglycerol mimetics (TGMs) we have designed (TGM0 and TGM5) markedly enhance GBE1Y329S activity, possibly enough for reversing APBD symptoms. We show that the GBE1Y329S mutation exposes a hydrophobic amino acid stretch, which can either stabilize and enhance or alternatively, reduce the enzyme activity via alteration of protein-membrane interactions. Additionally, we found that WT, but not Y329S, GBE1 activity is modulated by Ca2+ and phosphatidylserine, probably associated with GBE1-mediated regulation of energy consumption and storage. The thermal stabilization and increase in GBE1Y329S activity induced by TGM5 and its omega-3 oil structure suggest that this molecule has a considerable therapeutic potential for treating APBD.
Keywords: adult polyglucosan body disease; diseases; drug therapy; membrane lipid therapy; metabolic disease; protein-membrane interactions; triglycerides; triheptanoin.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Moses S. W., and Parvari R.. 2002. The variable presentations of glycogen storage disease type IV: a review of clinical, enzymatic and molecular studies. Curr. Mol. Med. 2: 177–188. - PubMed
-
- Lossos A., Meiner Z., Barash V., Soffer D., Schlesinger I., Abramsky O., Argov Z., Shpitzen S., and Meiner V.. 1998. Adult polyglucosan body disease in Ashkenazi Jewish patients carrying the Tyr329Ser mutation in the glycogen-branching enzyme gene. Ann. Neurol. 44: 867–872. - PubMed
-
- Hussain A., Armistead J., Gushulak L., Kruck C., Pind S., Triggs-Raine B., and Natowicz M. R.. 2012. The adult polyglucosan body disease mutation GBE1 c.1076A>C occurs at high frequency in persons of Ashkenazi Jewish background. Biochem. Biophys. Res. Commun. 426: 286–288. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

