Determination of the Residues in the Extracellular Domain of the Nicotinic α Subunit Required for the Actions of Physostigmine on Neuronal Nicotinic Receptors
- PMID: 28630263
- PMCID: PMC5548365
- DOI: 10.1124/mol.117.108894
Determination of the Residues in the Extracellular Domain of the Nicotinic α Subunit Required for the Actions of Physostigmine on Neuronal Nicotinic Receptors
Abstract
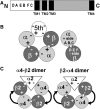
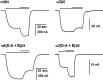
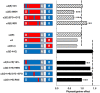
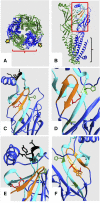
Physostigmine can potentiate and inhibit neuronal nicotinic receptors, in addition to inhibiting the activity of acetylcholinesterase. We found that receptors containing three copies of the α2 subunit are inhibited by low concentrations of physostigmine in contrast to receptors containing three copies of the α4 subunit that are potentiated. We exploited this observation to determine the regions required for the actions of physostigmine. Chimeric constructs of the α2 and α4 subunits located two regions in the extracellular amino-terminal domain of the subunit: the E loop (a loop of the transmitter-binding domain) and a region closer to the amino-terminus that collectively could completely determine the different effects of physostigmine. Point mutations then identified a single residue, α2(I92) versus α4(R92), that, when combined with transfer of the E loop, could convert the inhibition seen with α2 subunits to potentiation and the potentiation seen with α4 subunits to inhibition. In addition, other point mutations could affect the extent of potentiation or inhibition, indicating that a more extensive set of interactions in the amino-terminal domain plays some role in the actions of physostigmine.
Copyright © 2017 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

