Heat exchange between a bouncing drop and a superhydrophobic substrate
- PMID: 28630306
- PMCID: PMC5502603
- DOI: 10.1073/pnas.1700197114
Heat exchange between a bouncing drop and a superhydrophobic substrate
Abstract
The ability to enhance or limit heat transfer between a surface and impacting drops is important in applications ranging from industrial spray cooling to the thermal regulation of animals in cold rain. When these surfaces are micro/nanotextured and hydrophobic, or superhydrophobic, an impacting drop can spread and recoil over trapped air pockets so quickly that it can completely bounce off the surface. It is expected that this short contact time limits heat transfer; however, the amount of heat exchanged and precise role of various parameters, such as the drop size, are unknown. Here, we demonstrate that the amount of heat exchanged between a millimeter-sized water drop and a superhydrophobic surface will be orders of magnitude less when the drop bounces than when it sticks. Through a combination of experiments and theory, we show that the heat transfer process on superhydrophobic surfaces is independent of the trapped gas. Instead, we find that, for a given spreading factor, the small fraction of heat transferred is controlled by two dimensionless groupings of physical parameters: one that relates the thermal properties of the drop and bulk substrate and the other that characterizes the relative thermal, inertial, and capillary dynamics of the drop.
Keywords: droplets; feathers; heat transfer; microtexture; wetting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
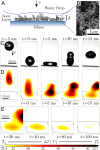









References
-
- Leon Bolle JCM. Spray cooling of hot surfaces. Multiphas Sci Tech. 1982;1:1–97.
-
- Kim J. Spray cooling heat transfer: The state of the art. Int J Heat Fluid Flow. 2007;28:753–767.
-
- Deng W, Gomez A. Electrospray cooling for microelectronics. Int J Heat Mass Tran. 2011;54:2270–2275.
-
- Pikkula BM, Torres JH, Tunnell JW, Anvari B. Cryogen spray cooling: Effects of droplet size and spray density on heat removal. Laser Surg Med. 2001;28:103–112. - PubMed
-
- Thomas SK, Cassoni RP, MacArthur CD. Aircraft anti-icing and de-icing techniques and modeling. J Aircraft. 1996;33:841–854.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

