Synthesis of asymmetrical multiantennary human milk oligosaccharides
- PMID: 28630345
- PMCID: PMC5502611
- DOI: 10.1073/pnas.1701785114
Synthesis of asymmetrical multiantennary human milk oligosaccharides
Abstract
Despite mammalian glycans typically having highly complex asymmetrical multiantennary architectures, chemical and chemoenzymatic synthesis has almost exclusively focused on the preparation of simpler symmetrical structures. This deficiency hampers investigations into the biology of glycan-binding proteins, which in turn complicates the biomedical use of this class of biomolecules. Herein, we describe an enzymatic strategy, using a limited number of human glycosyltransferases, to access a collection of 60 asymmetric, multiantennary human milk oligosaccharides (HMOs), which were used to develop a glycan microarray. Probing the array with several glycan-binding proteins uncovered that not only terminal glycoepitopes but also complex architectures of glycans can influence binding selectivity in unanticipated manners. N- and O-linked glycans express structural elements of HMOs, and thus, the reported synthetic principles will find broad applicability.
Keywords: chemoenzymatic synthesis; glycosyltransferases; human milk oligosaccharides; protein–glycan interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


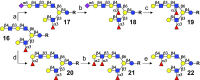
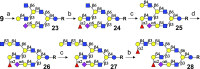


References
-
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. - PubMed
-
- Kunz C, Rudloff S. 2008. Potential anti-inflammatory and anti-infectious effects of human milk oligosaccharides. Bioactive Components of Milk, Advances in Experimental Medicine and Biology, ed Bösze Z (Springer, New York), Vol 606, pp 455–466.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

