Apical and basal epitheliomuscular F-actin dynamics during Hydra bud evagination
- PMID: 28630355
- PMCID: PMC5576072
- DOI: 10.1242/bio.022723
Apical and basal epitheliomuscular F-actin dynamics during Hydra bud evagination
Abstract
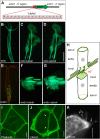
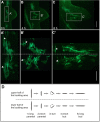
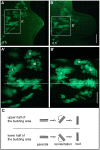
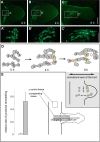
Bending of 2D cell sheets is a fundamental morphogenetic mechanism during animal development and reproduction. A critical player driving cell shape during tissue bending is the actin cytoskeleton. Much of our current knowledge about actin dynamics in whole organisms stems from studies of embryonic development in bilaterian model organisms. Here, we have analyzed actin-based processes during asexual bud evagination in the simple metazoan Hydra We created transgenic Hydra strains stably expressing the actin marker Lifeact-GFP in either ectodermal or endodermal epitheliomuscular cells. We then combined live imaging with conventional phalloidin staining to directly follow actin reorganization. Bending of the Hydra epithelial double layer is initiated by a group of epitheliomuscular cells in the endodermal layer. These cells shorten their apical-basal axis and arrange their basal muscle processes in a circular configuration. We propose that this rearrangement generates the initial forces to bend the endoderm towards the ectoderm. Convergent tissue movement in both epithelial layers towards the centre of evagination then leads to elongation and extension of the bud along its new body axis. Tissue movement into the bud is associated with lateral intercalation of epithelial cells, remodelling of apical septate junctions, and rearrangement of basal muscle processes. The work presented here extends the analysis of morphogenetic mechanisms beyond embryonic tissues of model bilaterians.
Keywords: Cnidarian; Epithelial cell; Evolution; Lifeact; Morphogenesis; Tissue evagination.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Campbell R. D. (1967). Cell behavior and morphogenesis in hydroids. In Vitro 3, 22-32. 10.1007/BF02615918 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

