Investigating the Life Expectancy and Proteolytic Degradation of Engineered Skeletal Muscle Biological Machines
- PMID: 28630410
- PMCID: PMC5476614
- DOI: 10.1038/s41598-017-03723-8
Investigating the Life Expectancy and Proteolytic Degradation of Engineered Skeletal Muscle Biological Machines
Abstract
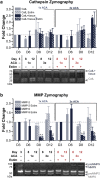
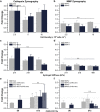
A combination of techniques from 3D printing, tissue engineering and biomaterials has yielded a new class of engineered biological robots that could be reliably controlled via applied signals. These machines are powered by a muscle strip composed of differentiated skeletal myofibers in a matrix of natural proteins, including fibrin, that provide physical support and cues to the cells as an engineered basement membrane. However, maintaining consistent results becomes challenging when sustaining a living system in vitro. Skeletal muscle must be preserved in a differentiated state and the system is subject to degradation by proteolytic enzymes that can break down its mechanical integrity. Here we examine the life expectancy, breakdown, and device failure of engineered skeletal muscle bio-bots as a result of degradation by three classes of proteases: plasmin, cathepsin L, and matrix metalloproteinases (MMP-2 and MMP-9). We also demonstrate the use of gelatin zymography to determine the effects of differentiation and inhibitor concentration on protease expression. With this knowledge, we are poised to design the next generation of complex biological machines with controllable function, specific life expectancy and greater consistency. These results could also prove useful for the study of disease-specific models, treatments of myopathies, and other tissue engineering applications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Three-dimensionally printed biological machines powered by skeletal muscle.Proc Natl Acad Sci U S A. 2014 Jul 15;111(28):10125-30. doi: 10.1073/pnas.1401577111. Epub 2014 Jun 30. Proc Natl Acad Sci U S A. 2014. PMID: 24982152 Free PMC article.
-
Optogenetic skeletal muscle-powered adaptive biological machines.Proc Natl Acad Sci U S A. 2016 Mar 29;113(13):3497-502. doi: 10.1073/pnas.1516139113. Epub 2016 Mar 14. Proc Natl Acad Sci U S A. 2016. PMID: 26976577 Free PMC article.
-
A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain.Acta Biomater. 2015 Sep;24:251-65. doi: 10.1016/j.actbio.2015.06.033. Epub 2015 Jun 30. Acta Biomater. 2015. PMID: 26141153
-
Assessment of skeletal muscle proteolysis and the regulatory response to nutrition and exercise.IUBMB Life. 2014 Jul;66(7):478-84. doi: 10.1002/iub.1291. Epub 2014 Jul 23. IUBMB Life. 2014. PMID: 25052691 Review.
-
Invited review: Muscle protein breakdown and its assessment in periparturient dairy cows.J Dairy Sci. 2023 Feb;106(2):822-842. doi: 10.3168/jds.2022-22068. Epub 2022 Nov 29. J Dairy Sci. 2023. PMID: 36460512 Review.
Cited by
-
Compliant 3D frameworks instrumented with strain sensors for characterization of millimeter-scale engineered muscle tissues.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2100077118. doi: 10.1073/pnas.2100077118. Proc Natl Acad Sci U S A. 2021. PMID: 33941674 Free PMC article.
-
Neuromuscular actuation of biohybrid motile bots.Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):19841-19847. doi: 10.1073/pnas.1907051116. Epub 2019 Sep 16. Proc Natl Acad Sci U S A. 2019. PMID: 31527266 Free PMC article.
-
Cysteine cathepsins are altered by flow within an engineered in vitro microvascular niche.APL Bioeng. 2020 Nov 4;4(4):046102. doi: 10.1063/5.0023342. eCollection 2020 Dec. APL Bioeng. 2020. PMID: 33195960 Free PMC article.
-
A forward-engineered, muscle-driven soft robotic swimmer.Sci Adv. 2025 Jul 18;11(29):eadu8634. doi: 10.1126/sciadv.adu8634. Epub 2025 Jul 16. Sci Adv. 2025. PMID: 40668908 Free PMC article.
-
Human cathepsins K, L, and S: Related proteases, but unique fibrinolytic activity.Biochim Biophys Acta Gen Subj. 2018 Sep;1862(9):1925-1932. doi: 10.1016/j.bbagen.2018.06.015. Epub 2018 Jun 23. Biochim Biophys Acta Gen Subj. 2018. PMID: 29944896 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

