Looking to nature for a new concept in antimicrobial treatments: isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA
- PMID: 28630440
- PMCID: PMC5476642
- DOI: 10.1038/s41598-017-03716-7
Looking to nature for a new concept in antimicrobial treatments: isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA
Abstract
The spread of multidrug-resistant Staphylococcus aureus strains, including methicillin-resistant S. aureus (MRSA), has shortened the useful life of anti-staphylococcal drugs enormously. Two approaches can be followed to address this problem: screening various sources for new leads for antibiotics or finding ways to disable the resistance mechanisms to existing antibiotics. Plants are resistant to most microorganisms, but despite extensive efforts to identify metabolites that are responsible for this resistance, no substantial progress has been made. Plants possibly use multiple strategies to deal with microorganisms that evolved over time. For this reason, we searched for plants that could potentiate the effects of known antibiotics. From 29 plant species tested, Cytisus striatus clearly showed such an activity and an NMR-based metabolomics study allowed the identification of compounds from the plant extracts that could act as antibiotic adjuvants. Isoflavonoids were found to potentiate the effect of ciprofloxacin and erythromycin against MRSA strains. For the structure-activity relationship (SAR), 22 isoflavonoids were assessed as antibiotic adjuvants. This study reveals a clear synergy between isoflavonoids and the tested antibiotics, showing their great potential for applications in the clinical therapy of infections with antibiotic-resistant microorganisms such as MRSA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
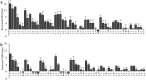
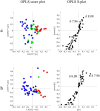
 , 2: additive
, 2: additive  and 3: potentiation effect
and 3: potentiation effect  ) and 1H-NMR data in the range of the region between δ 6.00 and 8.60 of the different classes of potentiating activity of extracts of C. striatus on ciprofloxacin (a) and erythromycin (b). The sample preparation and extraction conditions were performed as shown in Supplementary Table 2.
) and 1H-NMR data in the range of the region between δ 6.00 and 8.60 of the different classes of potentiating activity of extracts of C. striatus on ciprofloxacin (a) and erythromycin (b). The sample preparation and extraction conditions were performed as shown in Supplementary Table 2.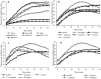
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

